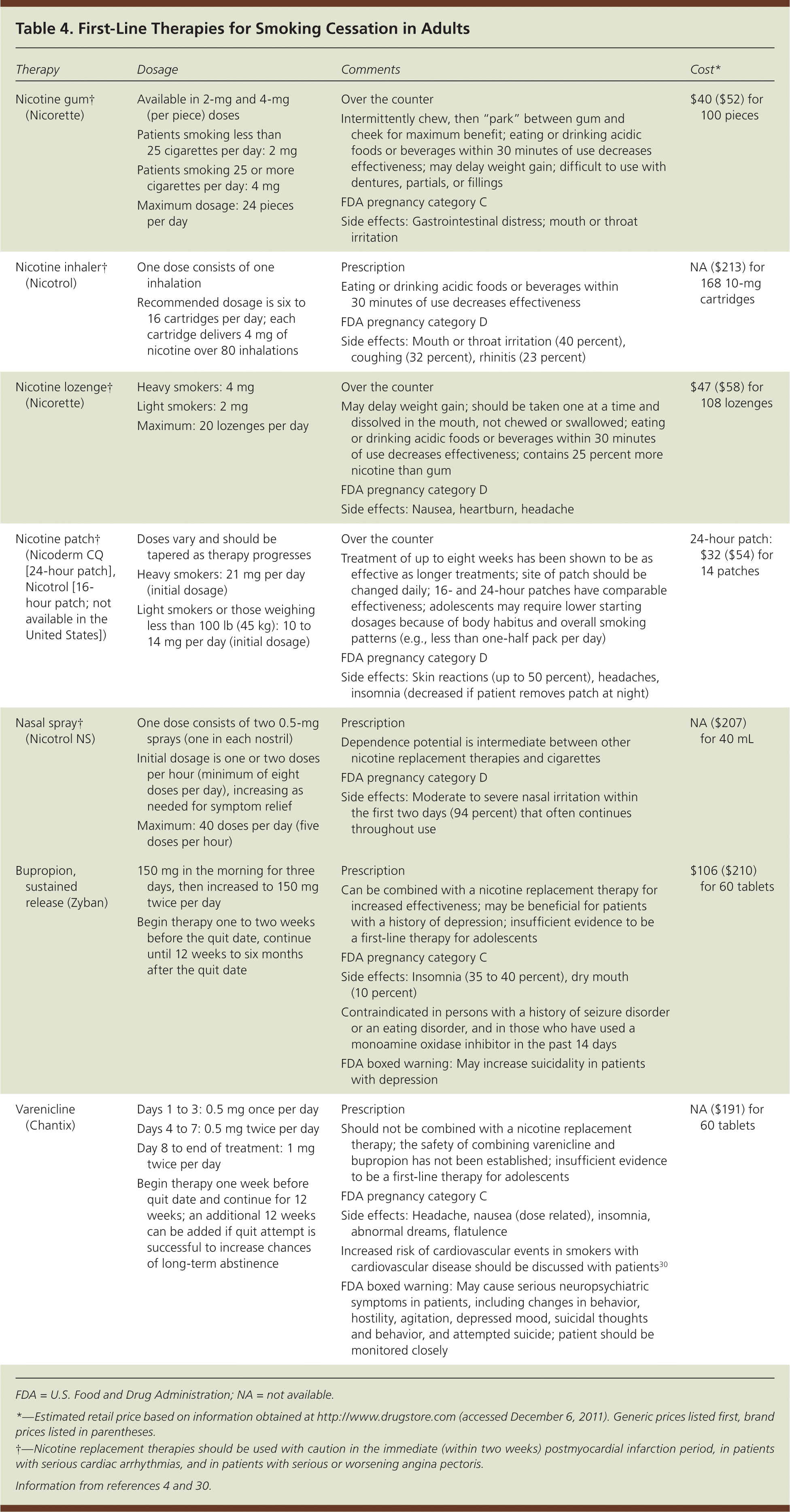
| Nicotine gum† (Nicorette) | Available in 2-mg and 4-mg (per piece) doses Patients smoking less than 25 cigarettes per day: 2 mg Patients smoking 25 or more cigarettes per day: 4 mg Maximum dosage: 24 pieces per day
| Over the counter Intermittently chew, then “park” between gum and cheek for maximum benefit; eating or drinking acidic foods or beverages within 30 minutes of use decreases effectiveness; may delay weight gain; difficult to use with dentures, partials, or fillings FDA pregnancy category C Side effects: Gastrointestinal distress; mouth or throat irritation
| $40 ($52) for 100 pieces |
| Nicotine inhaler† (Nicotrol) | | Prescription Eating or drinking acidic foods or beverages within 30 minutes of use decreases effectiveness FDA pregnancy category D Side effects: Mouth or throat irritation (40 percent), coughing (32 percent), rhinitis (23 percent)
| NA ($213) for 168 10-mg cartridges |
| Nicotine lozenge† (Nicorette) | | Over the counter May delay weight gain; should be taken one at a time and dissolved in the mouth, not chewed or swallowed; eating or drinking acidic foods or beverages within 30 minutes of use decreases effectiveness; contains 25 percent more nicotine than gum FDA pregnancy category D Side effects: Nausea, heartburn, headache
| $47 ($58) for 108 lozenges |
| Nicotine patch† (Nicoderm CQ [24-hour patch], Nicotrol [16-hour patch; not available in the United States]) | Doses vary and should be tapered as therapy progresses Heavy smokers: 21 mg per day (initial dosage) Light smokers or those weighing less than 100 lb (45 kg): 10 to14 mg per day (initial dosage)
| Over the counter Treatment of up to eight weeks has been shown to be as effective as longer treatments; site of patch should be changed daily; 16- and 24-hour patches have comparable effectiveness; adolescents may require lower starting dosages because of body habitus and overall smoking patterns (e.g., less than one-half pack per day) FDA pregnancy category D Side effects: Skin reactions (up to 50 percent), headaches, insomnia (decreased if patient removes patch at night)
| 24-hour patch: $32 ($54) for 14 patches |
| Nasal spray† (Nicotrol NS) | One dose consists of two 0.5-mg sprays (one in each nostril) Initial dosage is one or two doses per hour (minimum of eight doses per day), increasing as needed for symptom relief Maximum: 40 doses per day (five doses per hour)
| Prescription Dependence potential is intermediate between other nicotine replacement therapies and cigarettes FDA pregnancy category D Side effects: Moderate to severe nasal irritation within the first two days (94 percent) that often continues throughout use
| NA ($207) for 40 mL |
| Bupropion, sustained release (Zyban) | 150 mg in the morning for three days, then increased to 150 mg twice per day Begin therapy one to two weeks before the quit date, continue until 12 weeks to six months after the quit date
| Prescription Can be combined with a nicotine replacement therapy for increased effectiveness; may be beneficial for patients with a history of depression; insufficient evidence to be a first-line therapy for adolescents FDA pregnancy category C Side effects: Insomnia (35 to 40 percent), dry mouth (10 percent) Contraindicated in persons with a history of seizure disorder or an eating disorder, and in those who have used a monoamine oxidase inhibitor in the past 14 days FDA boxed warning: May increase suicidality in patients with depression
| $106 ($210) for 60 tablets |
| Varenicline (Chantix) | Days 1 to 3: 0.5 mg once per day Days 4 to 7: 0.5 mg twice per day Day 8 to end of treatment: 1 mg twice per day Begin therapy one week before quit date and continue for 12 weeks; an additional 12 weeks can be added if quit attempt is successful to increase chances of long-term abstinence
| Prescription Should not be combined with a nicotine replacement therapy; the safety of combining varenicline and bupropion has not been established; insufficient evidence to be a first-line therapy for adolescents FDA pregnancy category C Side effects: Headache, nausea (dose related), insomnia, abnormal dreams, flatulence Increased risk of cardiovascular events in smokers with cardiovascular disease should be discussed with patients30 FDA boxed warning: May cause serious neuropsychiatric symptoms in patients, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, and attempted suicide; patient should be monitored closely
| NA ($191) for 60 tablets |