Am Fam Physician. 2013;87(3):211-212
Author disclosure: No relevant financial affiliations to disclose.
Fidaxomicin (Dificid) is an oral macrolide antibiotic labeled for the treatment of Clostridium difficile–associated diarrhea in adults. It primarily exhibits activity against species of clostridia, mainly C. difficile, while having no or limited activity against normal gastrointestinal flora. It joins the standard treatments of oral metronidazole (Flagyl) and oral vancomycin.
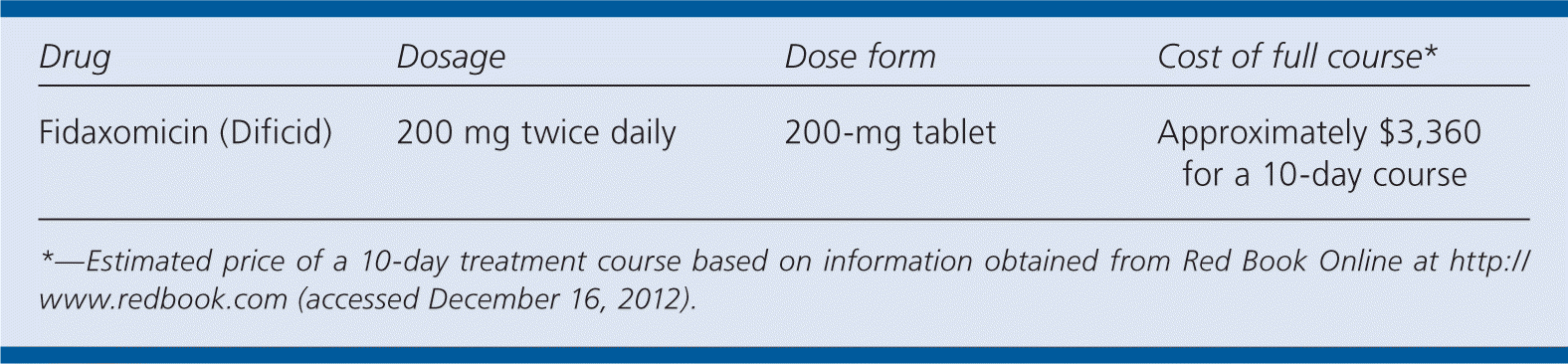
| Drug | Dosage | Dose form | Cost of full course* |
|---|---|---|---|
| Fidaxomicin (Dificid) | 200 mg twice daily | 200-mg tablet | Approximately $3,360 for a 10-day course |
SAFETY
Fidaxomicin is poorly absorbed; therefore, serious adverse effects are rare. There are no known drug-drug interactions or contraindications with use. Fidaxomicin should not be used for systemic infections, and should be used only for infections proven or strongly suspected to be caused by C. difficile. Fidaxomicin is a U.S. Food and Drug Administration pregnancy category B drug; it is not known whether it is excreted in human milk.1
TOLERABILITY
Fidaxomicin has an adverse effect profile comparable to that of oral vancomycin. In clinical trials of 564 patients, approximately 6 percent withdrew because of adverse effects. The most common adverse effects (greater than 5 percent) were nausea, vomiting, and abdominal pain.1
EFFECTIVENESS
Fidaxomicin has been compared exclusively with oral vancomycin in clinical trials for initial occurrences of C. difficile–associated diarrhea. In two studies enrolling a total of 1,057 participants, fidaxomicin (200 mg twice daily for 10 days) was as effective as vancomycin (125 mg every six hours for 10 days) at producing clinical cure, which was defined as the resolution of diarrhea (fewer than three unformed stools for two consecutive days).2,3 Fidaxomicin decreases recurrence of C. difficile infection compared with vancomycin (number needed to treat [NNT] = 7 to 10), although it is no more effective in patients who are infected with a hypervirulent strain of C. difficile that is more likely to cause complications and recurrence. Similarly, both are equally effective in treating a first recurrence, with fidaxomicin perhaps being slightly more effective in preventing a second recurrence (NNT = 3 to 333).4 Fidaxomicin has not been compared with metronidazole, and has not been studied in patients in whom treatment with metronidazole failed. All patients who have been studied have had confirmed cases of C. difficile infection; it has not been studied in patients with other causes of antibiotic-associated diarrhea.
PRICE
A 10-day supply of fidaxomicin will cost approximately $3,360. It is significantly more expensive than metronidazole (about $35), commercially available oral vancomycin ($700), or oral vancomycin compounded from intravenous vancomycin ($25).5
SIMPLICITY
Patients 18 years and older should be prescribed 200 mg of fidaxomicin twice daily for 10 days, to be taken with or without food. No dosage adjustments are necessary for older adults or for persons with renal impairment. No specific dosage recommendations exist for children or for patients who have hepatic impairment.
Bottom Line
Fidaxomicin is effective for the treatment of C. difficile infection, but it is much more expensive than traditional therapy. Until it is known that the benefits outweigh the significant cost, metronidazole or oral vancomycin is the preferred treatment for most patients.