
Am Fam Physician. 2013;87(10):718-720
Author disclosure: No relevant financial affiliations.
Bromocriptine mesylate (Cycloset) is an oral dopamine receptor agonist labeled for the treatment of type 2 diabetes mellitus. The mechanism of action is unknown, and improvement in glycemic control does not occur by increasing plasma insulin concentrations. Cycloset is formulated as a quick-release tablet, and is not interchangeable with other bromocriptine products.1
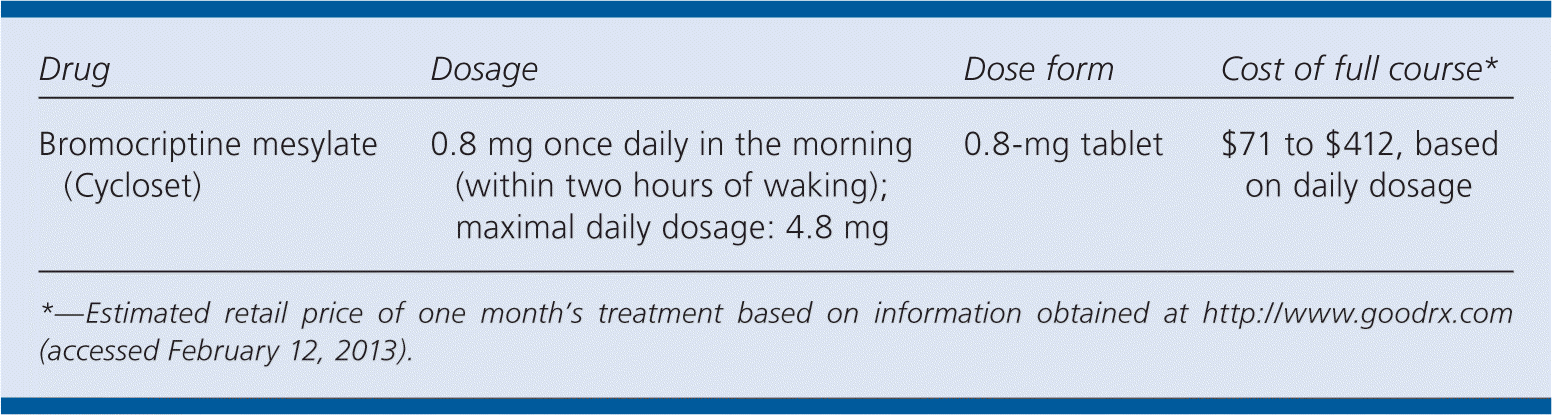
| Drug | Dosage | Dose form | Cost of full course* |
|---|---|---|---|
| Bromocriptine mesylate (Cycloset) | 0.8 mg once daily in the morning (within two hours of waking); maximal daily dosage: 4.8 mg | 0.8-mg tablet | $71 to $412, based on daily dosage |
SAFETY
Cycloset is not associated with an increased risk of cardiovascular events.2 Hypotension develops in about one in 50 patients. Persons treated concurrently with antihypertensive medications should be warned to report symptoms such as dizziness, nausea, or diaphoresis if they occur.1 Cycloset should not be used in patients who have a history of syncopal migraine. Hypoglycemia may occur in about 4 percent of patients when Cycloset is used alone, and in about 9 percent of patients when it is combined with a sulfonylurea. Severe hypoglycemia requiring medical treatment is uncommon. Cycloset has not been studied in children or patients with hepatic or renal insufficiency. It is a U.S. Food and Drug Administration pregnancy category B drug.
Cycloset is extensively metabolized by the cytochrome P450 3A4 liver enzyme system. Potent inhibitors (e.g., macrolide antibiotics, azole antifungals) or inducers (e.g., rifampin, carbamazepine [Tegretol], phenytoin [Dilantin]) of this enzyme may affect serum levels and possibly the action of Cycloset. Because its use with dopamine receptor antagonists (i.e., metoclopramide [Reglan], clozapine [Clozaril], and olanzapine [Zyprexa]) has not been studied, it should not be used concurrently with these agents. Cycloset is highly protein-bound, and can alter the effectiveness and increase adverse effects of salicylates, sulfonamides, chloramphenicol, and probenecid.
TOLERABILITY
About one in four patients will stop treatment with Cycloset because of adverse effects. Nausea, fatigue, dizziness, vomiting, and headaches can occur in up to one-third of patients during the first two weeks of treatment.1 It should be taken with food to reduce gastrointestinal symptoms.
EFFECTIVENESS
The effect of Cycloset on mortality or diabetes-related complications has not been evaluated. Based on studies of six months' duration, Cycloset will lower a patient's A1C level an average of 0.3 percentage points when used alone.2–4 When it is used in combination with sulfonylureas, an A1C decrease of 0.5 percentage points has been shown compared with use of sulfonylureas alone.3,4 When used with metformin (Glucophage) or insulin, Cycloset did not demonstrate any additional changes in A1C levels.2,5 The longer-term effects of Cycloset on glycemia markers are not known.
PRICE
The price of Cycloset varies depending on the dosage (0.8 to 4.8 mg per day). A one-month supply (2.4 mg per day) costs approximately $270.6 Metformin and sulfonylureas cost approximately $4 per month.
SIMPLICITY
Cycloset is available as an 0.8-mg tablet taken in the morning, within two hours of waking. The dosage can be increased at weekly intervals. Patients should take Cycloset with breakfast to minimize gastrointestinal effects. At the maximal dose, patients will need to take six tablets each morning. Aside from standard diabetes monitoring, no additional laboratory testing is needed.
Bottom Line
Cycloset will lower A1C levels by 0.3 to 0.5 percent when taken alone or in combination with a sulfonylurea, without undesirable adverse effects such as hypoglycemia and weight gain. It will not cause further improvement of glucose control when used with insulin, metformin, or other hypoglycemic agents. Whether it decreases mortality or diabetes-related morbidity is not known. For this reason, metformin is the preferred starting medication for most patients with type 2 diabetes.
