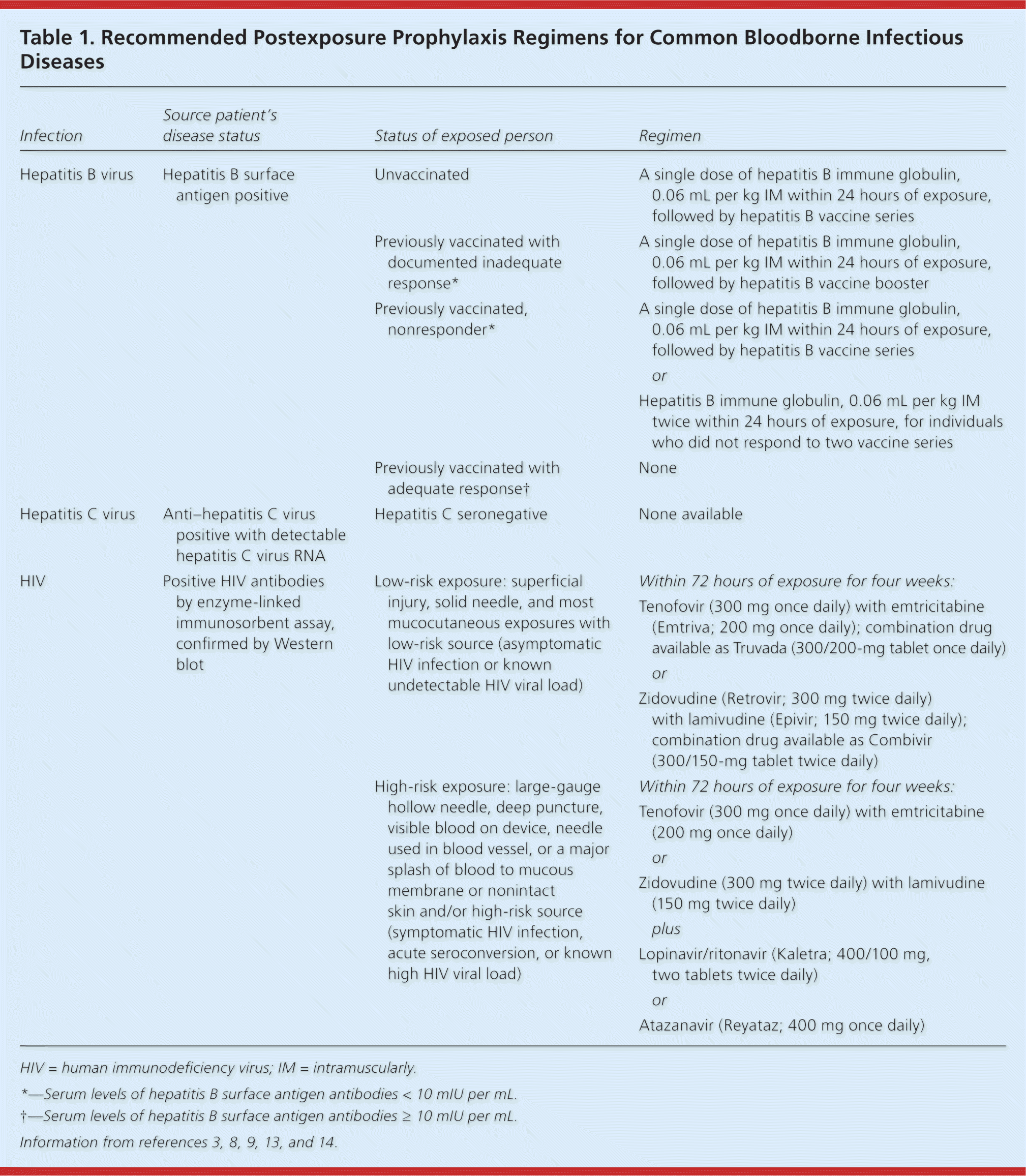
| Hepatitis B virus | Hepatitis B surface antigen positive | Unvaccinated | A single dose of hepatitis B immune globulin, 0.06 mL per kg IM within 24 hours of exposure, followed by hepatitis B vaccine series |
| Previously vaccinated with documented inadequate response* | A single dose of hepatitis B immune globulin, 0.06 mL per kg IM within 24 hours of exposure, followed by hepatitis B vaccine booster |
| Previously vaccinated, nonresponder* | A single dose of hepatitis B immune globulin, 0.06 mL per kg IM within 24 hours of exposure, followed by hepatitis B vaccine series |
| or |
| Hepatitis B immune globulin, 0.06 mL per kg IM twice within 24 hours of exposure, for individuals who did not respond to two vaccine series |
| Previously vaccinated with adequate response† | None |
| Hepatitis C virus | Anti–hepatitis C virus positive with detectable hepatitis C virus RNA | Hepatitis C seronegative | None available |
| HIV | Positive HIV antibodies by enzyme-linked immunosorbent assay, confirmed by Western blot | Low-risk exposure: superficial injury, solid needle, and most mucocutaneous exposures with low-risk source (asymptomatic HIV infection or known undetectable HIV viral load) | Within 72 hours of exposure for four weeks: |
| Tenofovir (300 mg once daily) with emtricitabine (Emtriva; 200 mg once daily); combination drug available as Truvada (300/200-mg tablet once daily) |
| or |
| Zidovudine (Retrovir; 300 mg twice daily) with lamivudine (Epivir; 150 mg twice daily); combination drug available as Combivir (300/150-mg tablet twice daily) |
| High-risk exposure: large-gauge hollow needle, deep puncture, visible blood on device, needle used in blood vessel, or a major splash of blood to mucous membrane or nonintact skin and/or high-risk source(symptomatic HIV infection, acute seroconversion, or known high HIV viral load) | Within 72 hours of exposure for four weeks: |
| Tenofovir (300 mg once daily) with emtricitabine (200 mg once daily) |
| or |
| Zidovudine (300 mg twice daily) with lamivudine (150 mg twice daily) |
| plus |
| Lopinavir/ritonavir (Kaletra; 400/100 mg, two tablets twice daily) |
| or |
| Atazanavir (Reyataz; 400 mg once daily) |