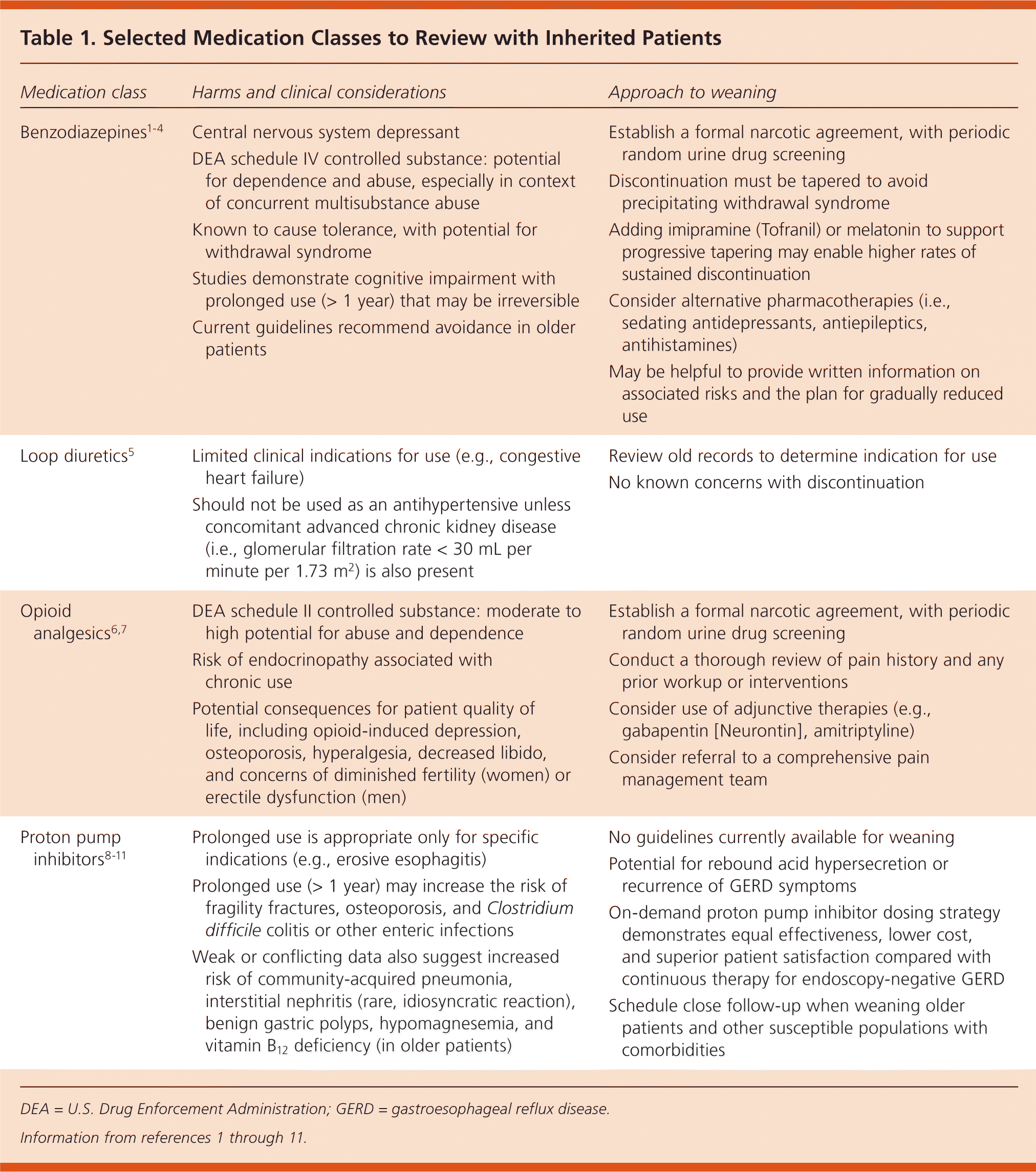
| Benzodiazepines1–4 | Central nervous system depressant DEA schedule IV controlled substance: potential for dependence and abuse, especially in context of concurrent multisubstance abuse Known to cause tolerance, with potential for withdrawal syndrome Studies demonstrate cognitive impairment with prolonged use (> 1 year) that may be irreversible Current guidelines recommend avoidance in older patients
| Establish a formal narcotic agreement, with periodic random urine drug screening Discontinuation must be tapered to avoid precipitating withdrawal syndrome Adding imipramine (Tofranil) or melatonin to support progressive tapering may enable higher rates of sustained discontinuation Consider alternative pharmacotherapies (i.e., sedating antidepressants, antiepileptics, antihistamines) May be helpful to provide written information on associated risks and the plan for gradually reduced use
|
| Loop diuretics5 | Limited clinical indications for use (e.g., congestive heart failure) Should not be used as an antihypertensive unless concomitant advanced chronic kidney disease (i.e., glomerular filtration rate < 30 mL per minute per 1.73 m2) is also present
| |
| Opioid analgesics6,7 | DEA schedule II controlled substance: moderate to high potential for abuse and dependence Risk of endocrinopathy associated with chronic use Potential consequences for patient quality of life, including opioid-induced depression, osteoporosis, hyperalgesia, decreased libido, and concerns of diminished fertility (women) or erectile dysfunction (men)
| Establish a formal narcotic agreement, with periodic random urine drug screening Conduct a thorough review of pain history and any prior workup or interventions Consider use of adjunctive therapies (e.g., gabapentin [Neurontin], amitriptyline) Consider referral to a comprehensive pain management team
|
| Proton pump inhibitors8–11 | Prolonged use is appropriate only for specific indications (e.g., erosive esophagitis) Prolonged use (> 1 year) may increase the risk of fragility fractures, osteoporosis, and Clostridium difficile colitis or other enteric infections Weak or conflicting data also suggest increased risk of community-acquired pneumonia, interstitial nephritis (rare, idiosyncratic reaction), benign gastric polyps, hypomagnesemia, and vitamin B12 deficiency (in older patients)
| No guidelines currently available for weaning Potential for rebound acid hypersecretion or recurrence of GERD symptoms On-demand proton pump inhibitor dosing strategy demonstrates equal effectiveness, lower cost, and superior patient satisfaction compared with continuous therapy for endoscopy-negative GERD Schedule close follow-up when weaning older patients and other susceptible populations with comorbidities
|