Am Fam Physician. 2014;89(4):300-301
Author disclosure: No relevant financial affiliations.
Roflumilast (Daliresp) is an oral selective phosphodiesterase-4 (PDE4) inhibitor similar to theophylline, a nonselective phosphodiesterase inhibitor. It is labeled only for reducing exacerbations of chronic obstructive pulmonary disease (COPD) in patients with severe COPD associated with chronic bronchitis and previous exacerbations. The exact mechanism of action of roflumilast is unknown. It is not a bronchodilator, but its effect on PDE4 may decrease inflammation.1
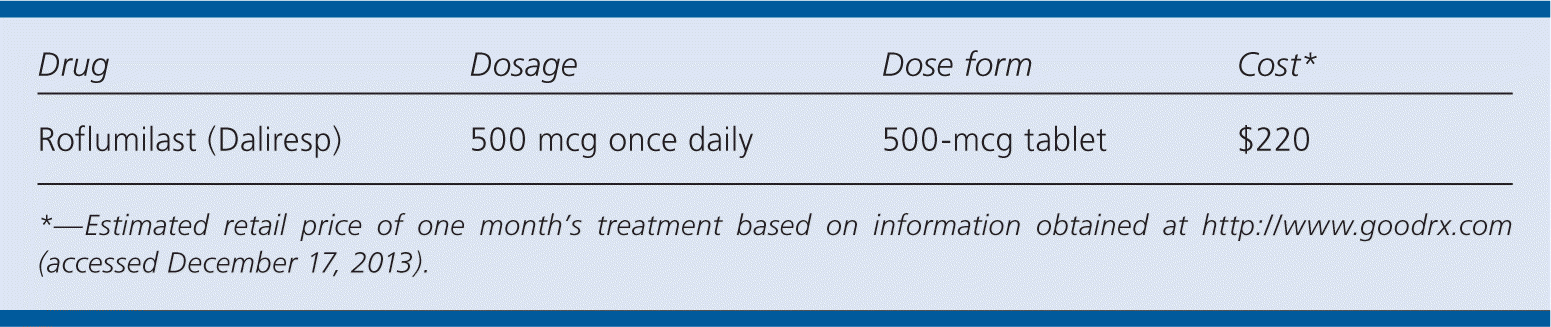
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Roflumilast (Daliresp) | 500 mcg once daily | 500-mcg tablet | $220 |
SAFETY
Roflumilast has been studied only in patients who have taken it for up to 52 weeks.2,3 Psychiatric effects, including suicidality, have been reported with roflumilast. In premarketing studies, suicide was attempted by five of 6,474 patients taking roflumilast and was completed by three of them. In the placebo group (n = 5,491), only one patient reported suicidal ideation.1,4 Depression, anxiety, and insomnia occur about twice as often in patients taking roflumilast as in those taking placebo. Roflumilast is not contraindicated in patients with a history of depression or suicidal ideation, but it makes sense to avoid use in these patients.
About two out of three patients may experience some level of weight loss on roflumilast, most commonly patients who are underweight before starting the medication and those with severe COPD.2,4 The average weight loss is about 4.7 lb (2.1 kg), although about one in four patients will lose more than 5% of his or her body weight.1,2 Most of the weight will be regained when the medication is discontinued. Underweight patients on roflumilast should be weighed regularly (about every four weeks) and treatment discontinued if clinically significant weight loss occurs.1,2,5
Blood levels of roflumilast may decrease when it is combined with strong cytochrome P450 (CYP450) inducers such as phenytoin (Dilantin) and carbamazepine (Tegretol), so these medications should not be used concomitantly. Conversely, it should be used cautiously with CYP450 inhibitors such as erythromycin, ketoconazole, and cimetidine (Tagamet) because side effects may be intensified. Though roflumilast does not interact with theophylline, these drugs should not be used together because they inhibit the same PDE4 enzyme. Roflumilast is a U.S. Food and Drug Administration pregnancy category C drug.1 It should not be used to treat acute bronchospasm.
TOLERABILITY
EFFECTIVENESS
Roflumilast has not been studied as an add-on to maximal conventional therapy. Roflumilast will decrease the number of exacerbations requiring oral corticosteroids in patients with severe COPD, a history of exacerbation, and bronchitic symptoms.1,4 In premarketing studies, adding roflumilast to long-acting beta2 agonists or short-acting anticholinergics decreased the average number of moderate or severe episodes requiring corticosteroid treatment by 0.23 per patient per year.2 It did not decrease the number of exacerbations requiring hospitalization. It is not effective in patients with mild to moderate COPD and has not been shown to affect mortality rates or improve quality of life.1,4,6 Roflumilast does not significantly reduce exacerbation rates when added to tiotropium (Spiriva),5 but it may have a slight additive effect when combined with inhaled corticosteroids in patients with severe COPD.7 When roflumilast is added to salmeterol (Serevent) therapy, the evidence of additional benefit is conflicting.5,8
PRICE
Roflumilast costs approximately $220 for one month of treatment, which is similar to a 30-day supply of other COPD therapies, such as fluticasone/salmeterol 250/50 (Advair; $290), budesonide/formoterol (Symbicort; $225), and tiotropium ($305).9
SIMPLICITY
Roflumilast is a 500-mcg tablet taken daily without regard to mealtimes and, unlike theophylline, does not require drug level monitoring. No dosage adjustment is necessary in patients with renal impairment. Roflumilast should not be used in patients with moderate to severe hepatic impairment.1
Bottom Line
Roflumilast therapy has a limited role in patients with severe COPD, and no role in patients with mild to moderate COPD. It will not decrease the number of hospitalizations. It will slightly lower the number of exacerbations requiring oral corticosteroid treatment, but only in select patients (i.e., those with a combination of severe COPD, current bronchitic symptoms, and a previous exacerbation). Roflumilast has not been shown to affect mortality, daily symptoms, or quality of life, and it has not been studied in optimally treated patients. Its use is further limited by psychiatric effects, weight loss, and gastrointestinal symptoms.