
A more recent article on tuberculosis is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2014;89(11):889-896
Patient information: See related handout on tuberculosis, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Latent tuberculosis infection refers to an asymptomatic, nontransmissible infection with Mycobacterium tuberculosis, carrying a 5% to 10% lifetime risk of progressing to active disease. One-half of this risk occurs within the first two years after infection. High-risk groups include recent immigrants from high-incidence countries, health care professionals, persons living or working in institutional settings, and homeless persons. Risk factors for progression to active disease include immunodeficiency, recent exposure to tuberculosis, and chronic kidney disease requiring dialysis. Tuberculin skin testing has several limitations, including the need for multiple office visits and the potential for false-positive results in patients who have received the bacillus Calmette-Guérin vaccine or been exposed to environmental mycobacteria. Interferon-gamma release assays address these deficiencies but are limited by their cost and requirement for blood processing. Interferon-gamma release assays are preferred in immigrants exposed to bacillus Calmette-Guérin and in patients who are not likely to return for interpretation of skin test results. Tuberculin skin testing is preferred for children younger than five years. Active disease should be excluded before initiating treatment. The newest treatment option of 12 weekly doses of isoniazid and rifapentine has similar or better effectiveness than standard nine-month therapy with daily isoniazid. A four-month regimen of daily rifampin is another alternative.
Infection with Mycobacterium tuberculosis is transmitted by airborne droplets from patients with active respiratory disease.1 After the primary infection, tuberculosis (TB) can progress to active pulmonary disease (most common) or extra-pulmonary disease, or it can remain latent for part or all of the patient's life. About one-third of the world's population is thought to be infected with M. tuberculosis, including an estimated 10 to 15 million latent infections in the United States.2 There is a 5% to 10% lifetime risk of progression from latent TB to active disease.1 One-half of this progression occurs in the first two years after infection, with the remaining risk distributed over the rest of the life span.3 Risk factors for infection and for progression from latent to active disease are listed in Table 1.3–7 Because more than 80% of active TB cases in the United States arise from latent TB, prompt treatment is key to prevent active disease.3 Patients with latent TB are noninfectious and typically do not feel ill, so the only indication of latent infection is a positive screening test (Table 2).7
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Persons in high-risk populations should be screened for tuberculosis and treated, if necessary. | C | 3, 5, 9 |
| Persons in low-risk populations should not be screened for tuberculosis because of the potential for false-positive results. A decision to test is a decision to treat. | C | 3, 5 |
| The interferon-gamma release assay is the preferred screening method for tuberculosis in patients with a history of bacillus Calmette-Guérin vaccination and in those unlikely to return for interpretation of tuberculin skin test results. Skin testing is preferred in children younger than five years. | C | 9 |
| Twelve weekly doses of isoniazid and rifapentine (Priftin) administered under direct observation are as effective as a nine-month regimen of daily isoniazid, and may result in better patient compliance. | B | 34, 35 |
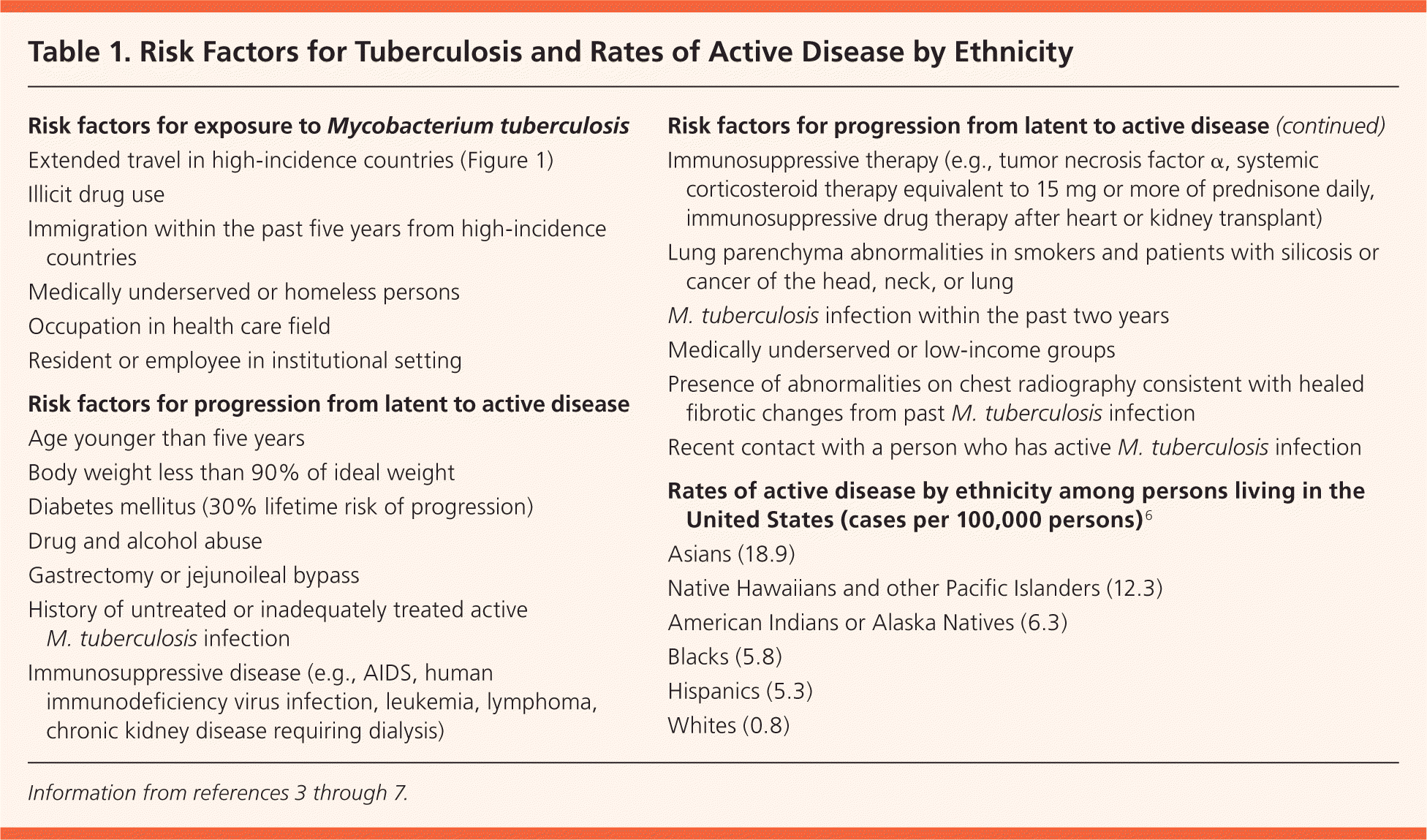
| Risk factors for exposure to Mycobacterium tuberculosis |
| Extended travel in high-incidence countries (Figure 1) |
| Illicit drug use |
| Immigration within the past five years from high-incidence countries |
| Medically underserved or homeless persons |
| Occupation in health care field |
| Resident or employee in institutional setting |
| Risk factors for progression from latent to active disease |
| Age younger than five years |
| Body weight less than 90% of ideal weight |
| Diabetes mellitus (30% lifetime risk of progression) |
| Drug and alcohol abuse |
| Gastrectomy or jejunoileal bypass |
| History of untreated or inadequately treated active M. tuberculosis infection |
| Immunosuppressive disease (e.g., AIDS, human immunodeficiency virus infection, leukemia, lymphoma, chronic kidney disease requiring dialysis) |
| Immunosuppressive therapy (e.g., tumor necrosis factor α, systemic corticosteroid therapy equivalent to 15 mg or more of prednisone daily, immunosuppressive drug therapy after heart or kidney transplant) |
| Lung parenchyma abnormalities in smokers and patients with silicosis or cancer of the head, neck, or lung |
| M. tuberculosis infection within the past two years |
| Medically underserved or low-income groups |
| Presence of abnormalities on chest radiography consistent with healed fibrotic changes from past M. tuberculosis infection |
| Recent contact with a person who has active M. tuberculosis infection |
| Rates of active disease by ethnicity among persons living in the United States (cases per 100,000 persons)6 |
| Asians (18.9) |
| Native Hawaiians and other Pacific Islanders (12.3) |
| American Indians or Alaska Natives (6.3) |
| Blacks (5.8) |
| Hispanics (5.3) |
| Whites (0.8) |
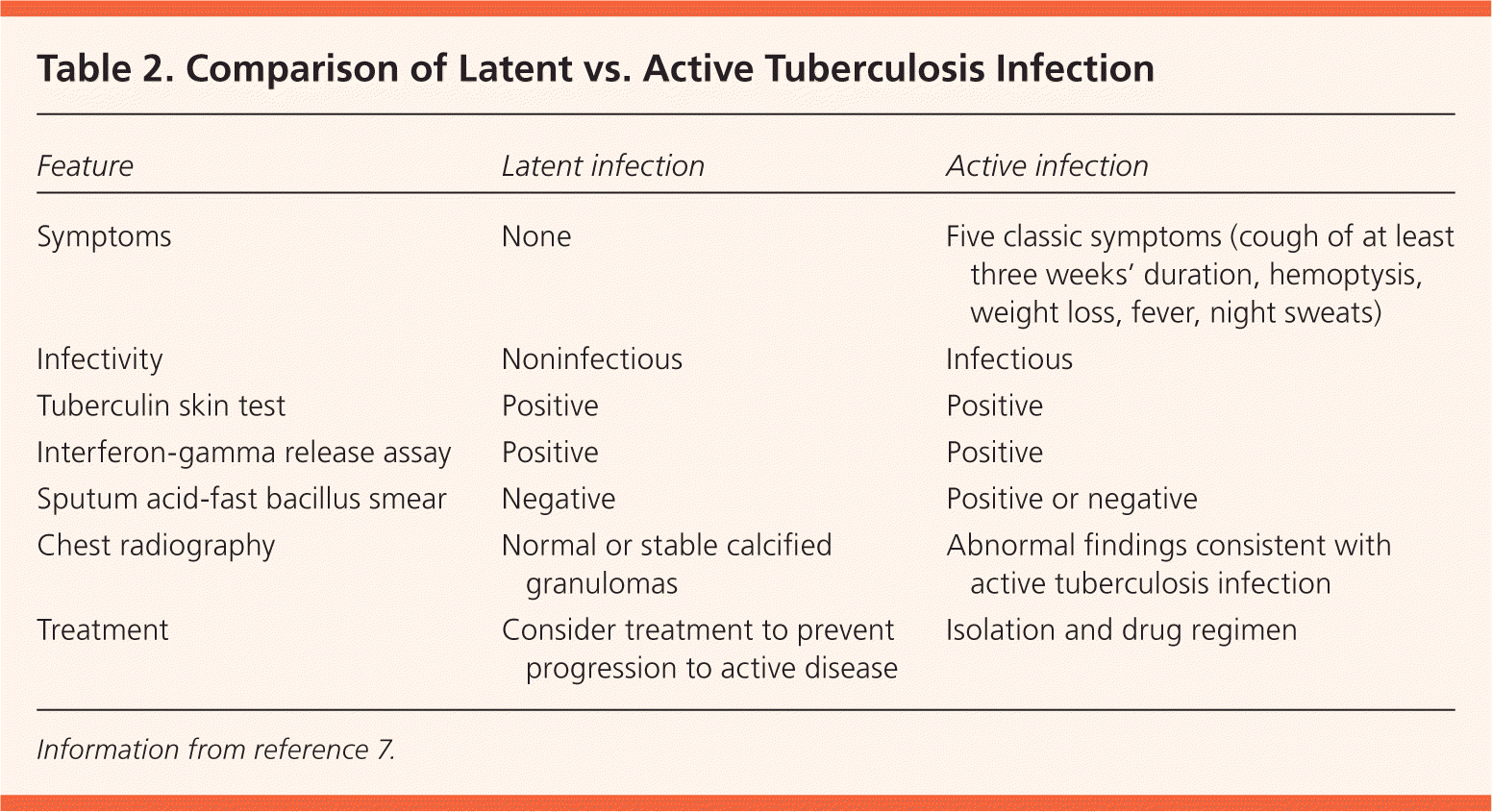
| Feature | Latent infection | Active infection |
|---|---|---|
| Symptoms | None | Five classic symptoms (cough of at least three weeks' duration, hemoptysis, weight loss, fever, night sweats) |
| Infectivity | Noninfectious | Infectious |
| Tuberculin skin test | Positive | Positive |
| Interferon-gamma release assay | Positive | Positive |
| Sputum acid-fast bacillus smear | Negative | Positive or negative |
| Chest radiography | Normal or stable calcified granulomas | Abnormal findings consistent with active tuberculosis infection |
| Treatment | Consider treatment to prevent progression to active disease | Isolation and drug regimen |
Risk Factors Warranting Testing
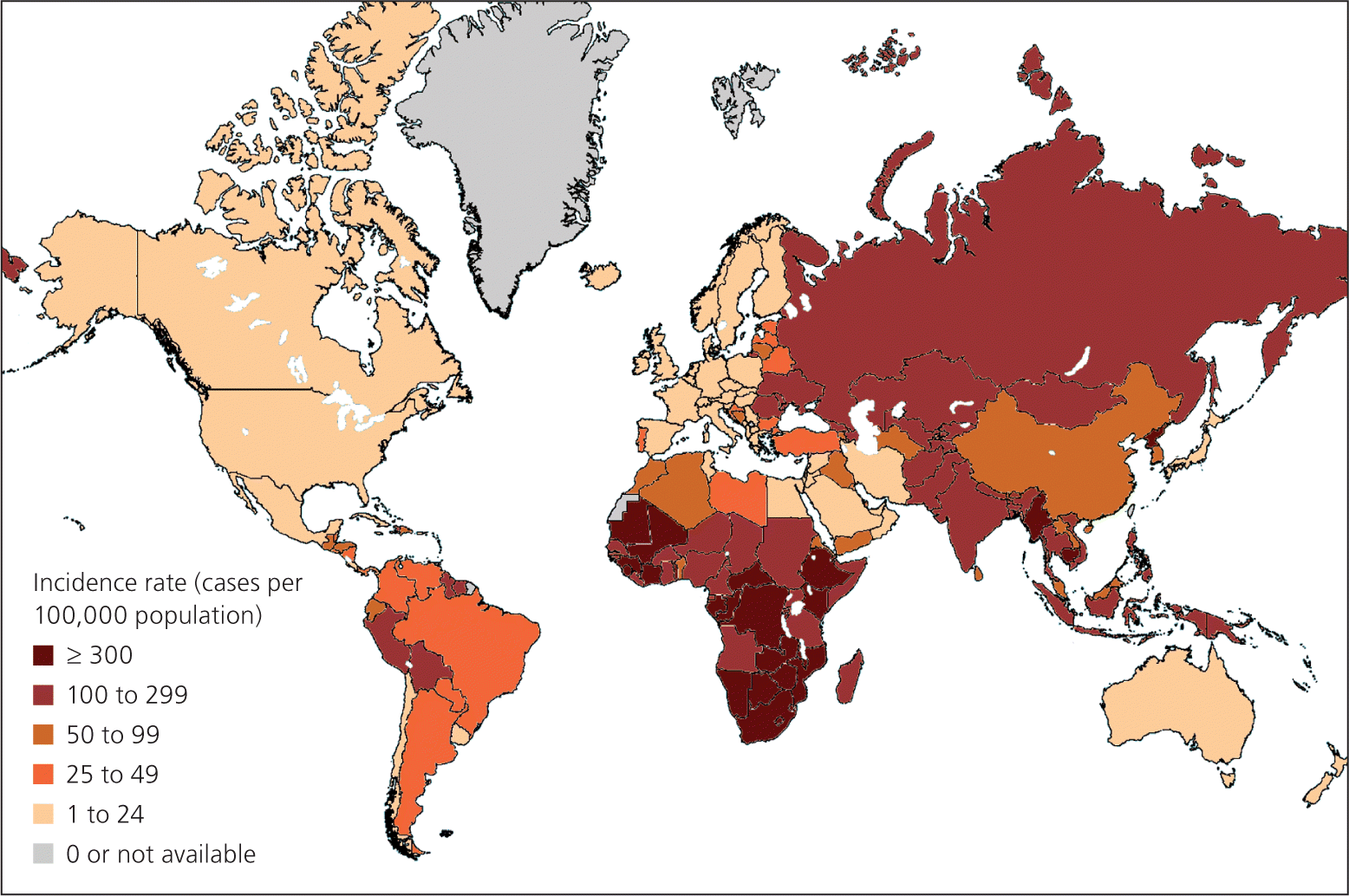
Targeted screening is recommended only for individuals and groups at increased risk of TB infection.5 Routine testing outside of high-risk groups leads to more false-positive results, creates needless anxiety, and wastes resources.5 High-risk groups include recent immigrants (within the past five years) from high-incidence countries, health care professionals, persons living or working in institutional settings, and homeless persons.4 High-incidence areas include most of the countries in Africa, Asia, eastern Europe, Central America, and South America (Figure 1).8 Asian immigrants in the United States are at significant risk, with 25 times the rate of active TB compared with non-Hispanic whites.9 The only low-risk persons who should be screened are those who will be entering a high-risk group in the future, such as for work or travel.5
Screening and Testing Options
Screening options for TB include the tuberculin skin test (TST) and interferon-gamma release assay (IGRA; Table 3).9,10 Although the IGRA is more specific and less sensitive than the TST, it does not eliminate the need for targeted testing in high-risk populations.11 Interpretation of both tests must be based on the patient's immune status, history of exposure to TB and bacillus Calmette-Guérin (BCG), and other risk factors.9 With the TST, an induration of 15 mm or more is considered positive in persons without risk factors, 10 mm or more is positive for those at higher risk, and 5 mm or more is positive for certain high-risk persons (e.g., immunocompromised patients, those exposed to active TB).12,13
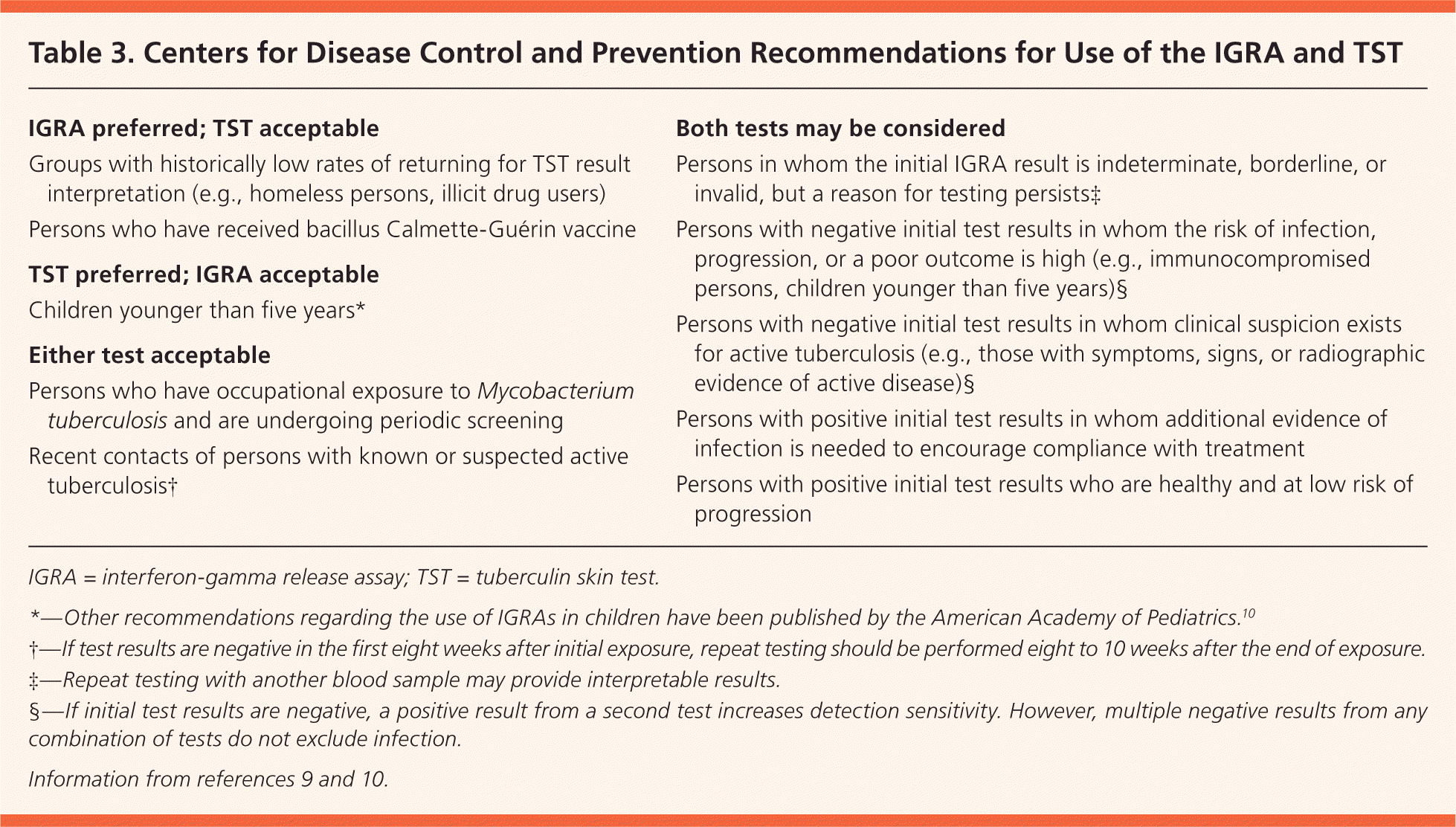
| IGRA preferred; TST acceptable |
| Groups with historically low rates of returning for TST result interpretation (e.g., homeless persons, illicit drug users) |
| Persons who have received bacillus Calmette-Guérin vaccine |
| TST preferred; IGRA acceptable |
| Children younger than five years* |
| Either test acceptable |
| Persons who have occupational exposure to Mycobacterium tuberculosis and are undergoing periodic screening |
| Recent contacts of persons with known or suspected active tuberculosis† |
| Both tests may be considered |
| Persons in whom the initial IGRA result is indeterminate, borderline, or invalid, but a reason for testing persists‡ |
| Persons with negative initial test results in whom the risk of infection, progression, or a poor outcome is high (e.g., immunocompromised persons, children younger than five years)§ |
| Persons with negative initial test results in whom clinical suspicion exists for active tuberculosis (e.g., those with symptoms, signs, or radiographic evidence of active disease)§ |
| Persons with positive initial test results in whom additional evidence of infection is needed to encourage compliance with treatment |
| Persons with positive initial test results who are healthy and at low risk of progression |
Use of the IGRA is preferred for persons who have received the BCG vaccine and those who are less likely to return for interpretation of TST results (e.g., homeless persons).9 Despite previous recommendations that TST results be interpreted without regard for BCG vaccination status, about 20% of positive TST results in this population may represent false positives rather than latent or active TB infection. False-positive results are more likely if the interval between BCG vaccination and use of the TST is less than 10 years.14 Therefore, the IGRA is preferred for immigrants who have received the BCG vaccine.9
The TST is preferred for children younger than five years9 because of reported variability and decreased sensitivity of IGRA testing in this age group.15 Children younger than five years are at higher risk of progression to more severe active disease, probably because of a lower interferon-gamma and T-cell response compared with older children and adults.16
Screening with the TST or IGRA may be performed after exposure to active TB. Either test may not show conversion for eight to 12 weeks in infected individuals, so “window prophylaxis” should be initiated in exposed high-risk patients, such as immunosuppressed persons or young children5 (Table 41,2,17–20 ). If repeat testing at 12 weeks is negative, medications can be stopped, although further clinical evaluation is indicated for immunosuppressed patients.9
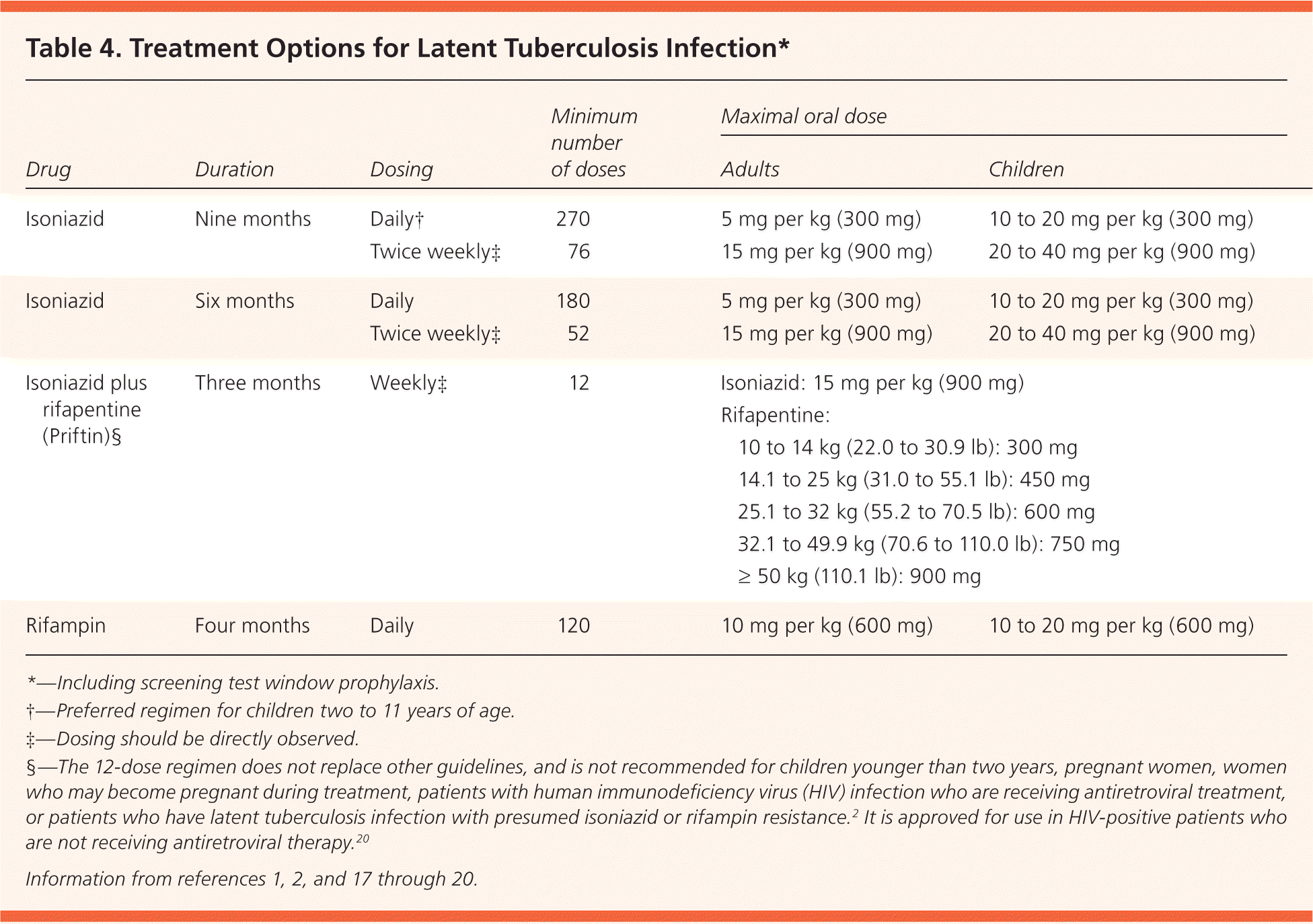
| Drug | Duration | Dosing | Minimum number of doses | Maximal oral dose | ||
|---|---|---|---|---|---|---|
| Adults | Children | |||||
| Isoniazid | Nine months | Daily† | 270 | 5 mg per kg (300 mg) | 10 to 20 mg per kg (300 mg) | |
| Twice weekly‡ | 76 | 15 mg per kg (900 mg) | 20 to 40 mg per kg (900 mg) | |||
| Isoniazid | Six months | Daily | 180 | 5 mg per kg (300 mg) | 10 to 20 mg per kg (300 mg) | |
| Twice weekly‡ | 52 | 15 mg per kg (900 mg) | 20 to 40 mg per kg (900 mg) | |||
| Isoniazid plus rifapentine (Priftin)§ | Three months | Weekly‡ | 12 | Isoniazid: 15 mg per kg (900 mg) | ||
Rifapentine:
| ||||||
| Rifampin | Four months | Daily | 120 | 10 mg per kg (600 mg) | 10 to 20 mg per kg (600 mg) | |
The TST or IGRA may be used for periodic screening of persons at risk of occupational exposure to M. tuberculosis. IGRA results are more likely to be positive in recently infected persons who are at highest risk of progression to active disease.21 IGRA results can be obtained after a single visit. Disadvantages of IGRA use include the processing requirements and higher cost.22 Tests can range from up to $25 at local health departments to $140 to $260 at a private reference laboratory.
The two-step TST involves an initial test and then a repeat test in one to three weeks to stimulate a reaction or booster effect in persons infected with M. tuberculosis in the distant past.13 When results of both the initial test and the repeat test are negative, a negative baseline is established. This process is often used for persons who may need frequent screening, such as health care professionals. If the first test is negative but the subsequent test is positive, infection should be assumed.
Discordant Test Results
Clinical judgment should be used when assessing discordant test results, taking into consideration the patient's risk of infection, of developing active disease, and of poor outcomes. The Centers for Disease Control and Prevention supports the use of the IGRA in addition to the TST in specific circumstances.9 It does not advocate confirmatory testing (IGRA to confirm positive TST results), unlike the United Kingdom, Switzerland, France, and Canada.23–25 Despite this, some U.S. health departments have adopted confirmatory IGRA. There is a 78.9% concordance rate between TST and IGRA results in healthy populations.17 Agreement is much more likely when TST results are negative (90.6% vs. 44.4% when positive).26 When results are discordant, TB risk factors and potential causes of false-positive and false-negative results should be considered (Table 5).5,9,12,17,22 The TST and IGRA measure different aspects of the immune response, with different interpretation criteria9 (Table 65,9,17,20,22,27 ). Most discordance in low-prevalence populations is related to these differences.28
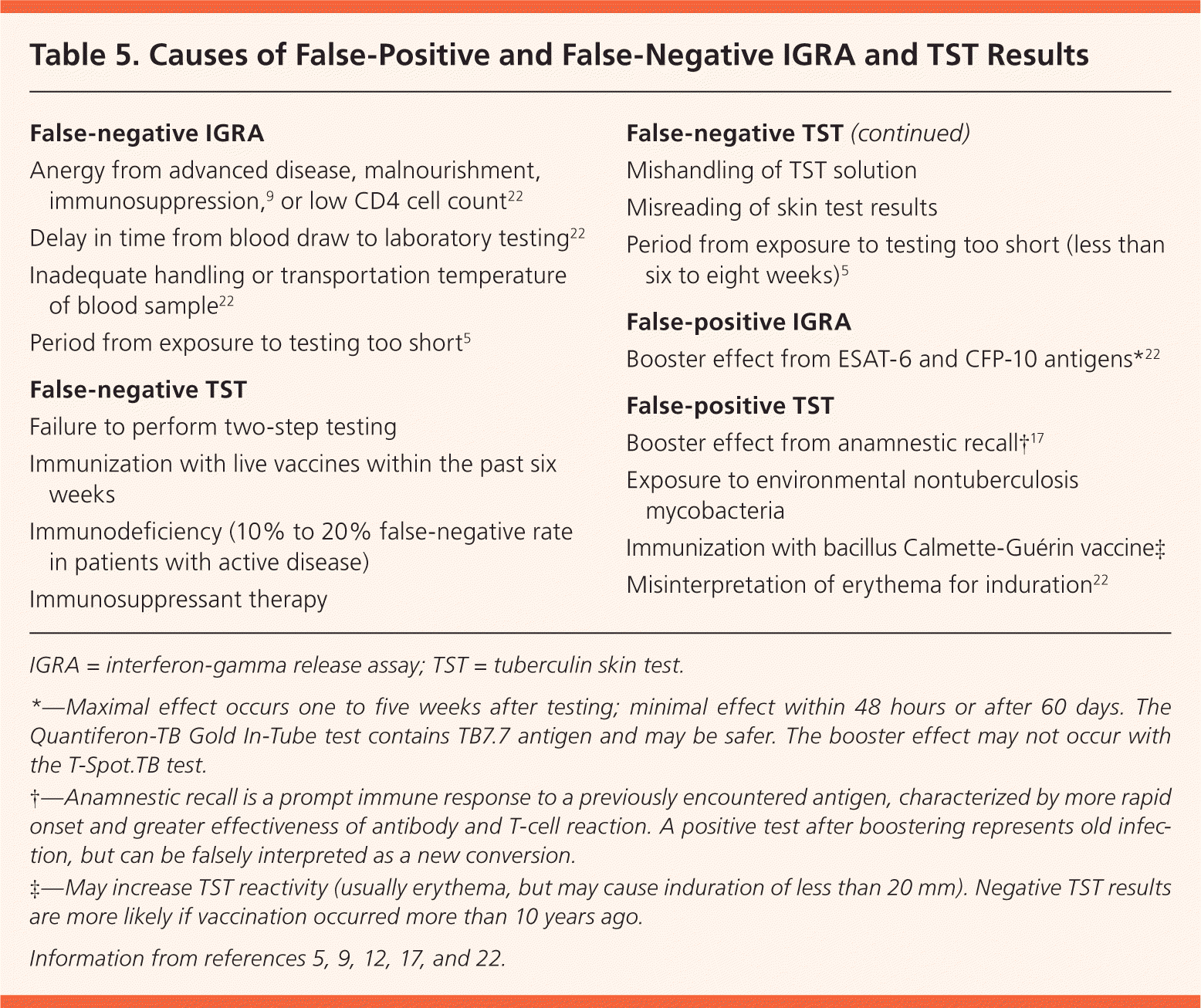
| False-negative IGRA |
| Anergy from advanced disease, malnourishment, immunosuppression,9 or low CD4 cell count22 |
| Delay in time from blood draw to laboratory testing22 |
| Inadequate handling or transportation temperature of blood sample22 |
| Period from exposure to testing too short5 |
| False-negative TST |
| Failure to perform two-step testing |
| Immunization with live vaccines within the past six weeks |
| Immunodeficiency (10% to 20% false-negative rate in patients with active disease) |
| Immunosuppressant therapy |
| Mishandling of TST solution |
| Misreading of skin test results |
| Period from exposure to testing too short (less than six to eight weeks)5 |
| False-positive IGRA |
| Booster effect from ESAT-6 and CFP-10 antigens*22 |
| False-positive TST |
| Booster effect from anamnestic recall†17 |
| Exposure to environmental nontuberculosis mycobacteria |
| Immunization with bacillus Calmette-Guérin vaccine‡ |
| Misinterpretation of erythema for induration22 |
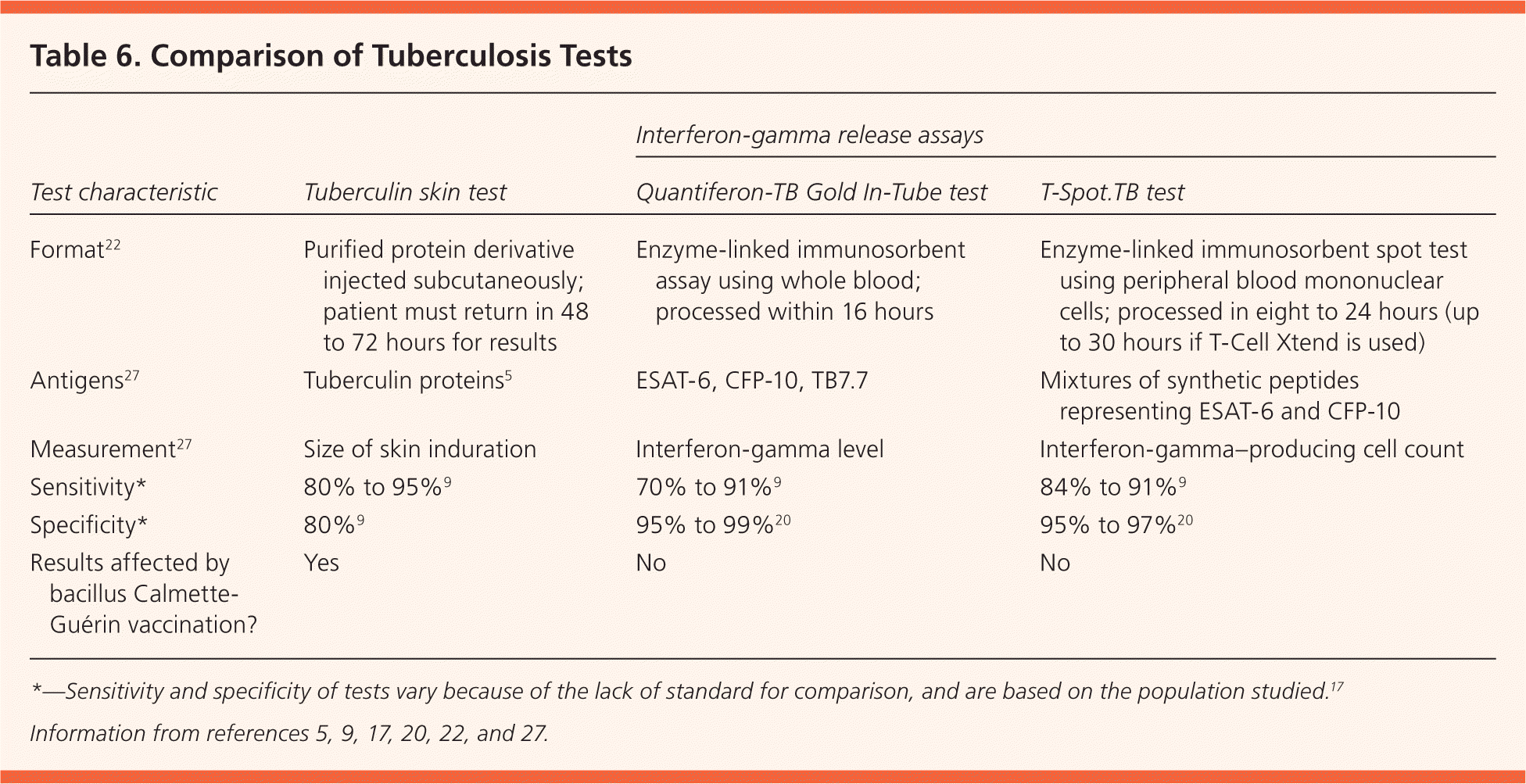
| Test characteristic | Tuberculin skin test | Interferon-gamma release assays | |
|---|---|---|---|
| Quantiferon-TB Gold In-Tube test | T-Spot.TB test | ||
| Format22 | Purified protein derivative injected intradermally; patient must return in 48 to 72 hours for results [ corrected] | Enzyme-linked immunosorbent assay using whole blood; processed within 16 hours | Enzyme-linked immunosorbent spot test using peripheral blood mononuclear cells; processed in eight to 24 hours (up to 30 hours if T-Cell Xtend is used) |
| Antigens27 | Tuberculin proteins5 | ESAT-6, CFP-10, TB7.7 | Mixtures of synthetic peptides representing ESAT-6 and CFP-10 |
| Measurement27 | Size of skin induration | Interferon-gamma level | Interferon-gamma–producing cell count |
| Sensitivity* | 80% to 95%9 | 70% to 91%9 | 84% to 91%9 |
| Specificity* | 80%9 | 95% to 99%20 | 95% to 97%20 |
| Results affected by bacillus Calmette-Guérin vaccination? | Yes | No | No |
Screening tests cannot detect the presence of M. tuberculosis; instead, they measure the host's immune response to past or current infection.9 The TST is based on delayed hypersensitivity to TB antigens. The current generation of IGRAs measures interferon-gamma released upon stimulation of T cells in response to the ESAT-6 and CFP-10 antigens of M. tuberculosis.5,9,29 Although sensitive, the TST has poor specificity, partly because of cross reactivity and interpretation errors.9 Estimating the sensitivity of the TST and IGRA is problematic because of the lack of a preferred test for latent TB infection.17 The IGRA is more specific (greater than 95%) with fewer false-positive results because the test antigens are not shared by BCG or environmental mycobacteria (except Mycobacterium marinum, Mycobacterium szulgai, and Mycobacterium kansasii).17,29 It should be noted that the TST contains ESAT-6 and CFP-10 antigens, which may boost IGRA results.22
Confirmatory testing may be considered in high-risk patients. Because of impaired immune response in these patients, negative TST results may be confirmed with an IGRA.9 Conversely, in low-risk patients, positive TST (or IGRA) results should not be considered proof of infection. Relying solely on positive TST results in low-risk patients could lead to unnecessary treatment, whereas additional IGRA testing could result in fewer treatment recommendations.22 IGRA confirmation of positive TST results may persuade reluctant patients to consider treatment.9
Treatment
Neither the TST nor the IGRA can distinguish between latent and active disease.17,20 At-risk patients with positive TB test results should be offered treatment after active disease is ruled out.5 A patient history should be obtained, and a physical examination and chest radiography should be performed. Any radiographic abnormalities should be followed with three sputum acid-fast bacillus smears to exclude active TB.
There are four approved treatment regimens for latent TB infection (Table 4).1,2,17–20 The standard nine-month isoniazid regimen reduces TB risk by 90% in patients who are fully compliant; however, only 64% of patients complete at least six months of therapy.3,30 A six-month regimen of isoniazid reduces risk by 60% to 80%.3 The less-studied four-month rifampin regimen is less expensive, has better patient compliance (69% to 78%), and has less liver toxicity31,32; four months of treatment provides 60% protection.33 A three-month, 12-dose regimen of isoniazid and rifapentine (Priftin), given once weekly under direct observation to ensure compliance and safety, is more expensive but as effective as nine months of isoniazid in persons 12 years and older.34 Its shorter course may result in better patient compliance.35 Monitoring recommendations and adverse effects of available regimens are listed in Table 7.1,5 As more evidence-based recommendations become available, the evaluation and treatment of latent TB infection will continue to evolve.
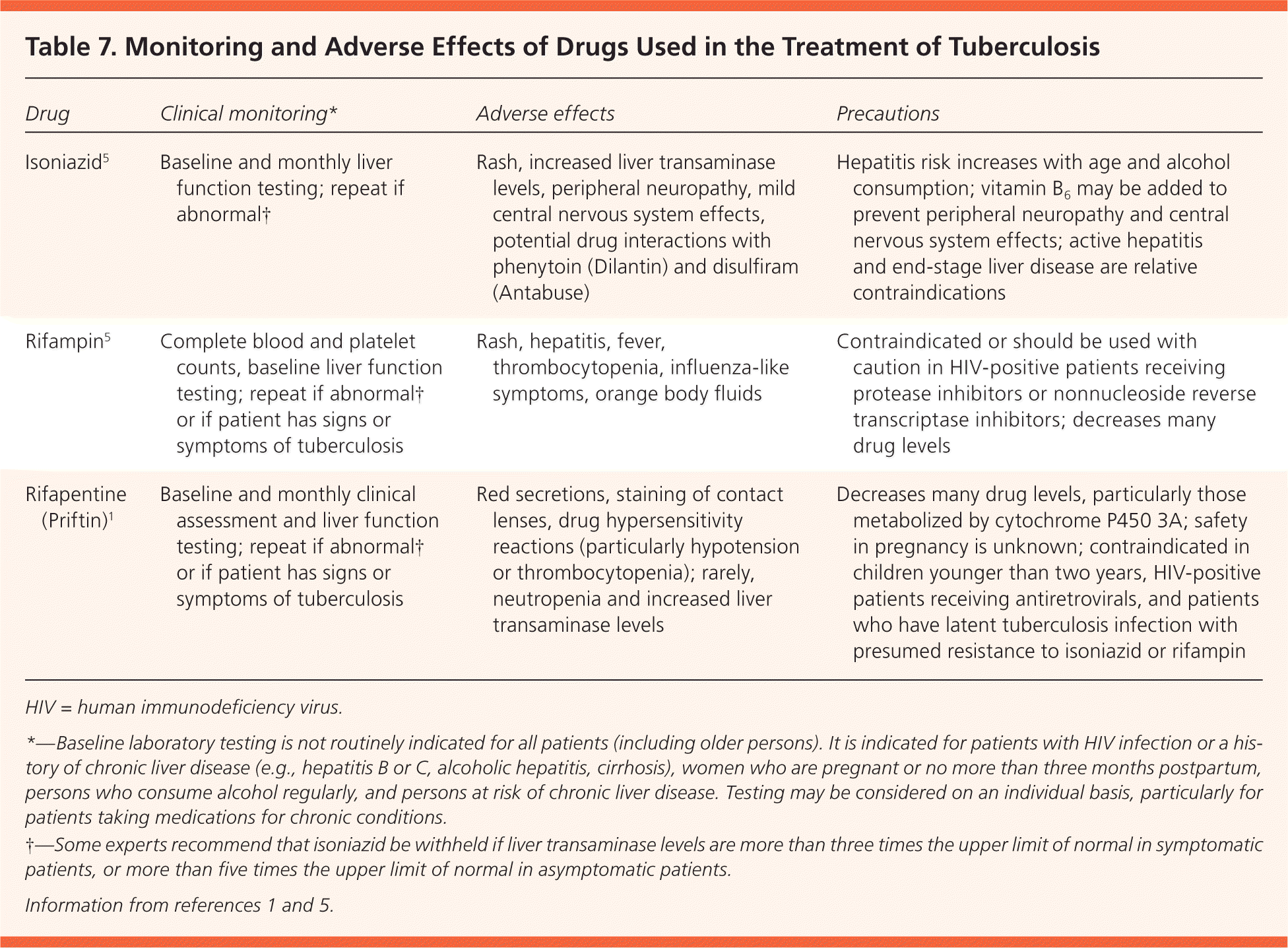
| Drug | Clinical monitoring* | Adverse effects | Precautions |
|---|---|---|---|
| Isoniazid5 | Baseline and monthly liver function testing; repeat if abnormal† | Rash, increased liver transaminase levels, peripheral neuropathy, mild central nervous system effects, potential drug interactions with phenytoin (Dilantin) and disulfiram (Antabuse) | Hepatitis risk increases with age and alcohol consumption; vitamin B6 may be added to prevent peripheral neuropathy and central nervous system effects; active hepatitis and end-stage liver disease are relative contraindications |
| Rifampin5 | Complete blood and platelet counts, baseline liver function testing; repeat if abnormal† or if patient has signs or symptoms of tuberculosis | Rash, hepatitis, fever, thrombocytopenia, influenza-like symptoms, orange body fluids | Contraindicated or should be used with caution in HIV-positive patients receiving protease inhibitors or nonnucleoside reverse transcriptase inhibitors; decreases many drug levels |
| Rifapentine (Priftin)1 | Baseline and monthly clinical assessment and liver function testing; repeat if abnormal† or if patient has signs or symptoms of tuberculosis | Red secretions, staining of contact lenses, drug hypersensitivity reactions (particularly hypotension or thrombocytopenia); rarely, neutropenia and increased liver transaminase levels | Decreases many drug levels, particularly those metabolized by cytochrome P450 3A; safety in pregnancy is unknown; contraindicated in children younger than two years, HIV-positive patients receiving antiretrovirals, and patients who have latent tuberculosis infection with presumed resistance to isoniazid or rifampin |
Data Sources: A PubMed search was completed in Clinical Queries using the key terms tuberculosis, exposure to tuberculosis, latent tuberculosis infection, TST and IGRA testing for tuberculosis, and treatment of tuberculosis. The search included meta-analyses, randomized controlled trials, and reviews. Also searched were the National Guideline Clearinghouse, Cochrane Database of Systematic Reviews, Centers for Disease Control and Prevention website, and Essential Evidence Plus. References from within those sources, as well as from UpToDate, were also searched. Search dates: January 20, 2012, to March 20, 2012, and January 24 to 30, 2014.
