A more recent article on acute pancreatitis is available.
Am Fam Physician. 2014;90(9):632-639
Patient information: A handout on this topic is available at https://familydoctor.org/familydoctor/en/diseases-conditions/pancreatitis.html.
Author disclosure: No relevant financial affiliations.
Acute pancreatitis is most commonly caused by gallstones or chronic alcohol use, and accounts for more than 200,000 hospital admissions annually. Using the Atlanta criteria, acute pancreatitis is diagnosed when a patient presents with two of three findings, including abdominal pain suggestive of pancreatitis, serum amylase and/or lipase levels at least three times the normal level, and characteristic findings on imaging. It is important to distinguish mild from severe disease because severe pancreatitis has a mortality rate of up to 30%. Contrast-enhanced computed tomography is considered the diagnostic standard for radiologic evaluation of acute pancreatitis because of its success in predicting disease severity and prognosis. The BALI and computed tomography severity index scores also can aid in determining disease severity and predicting the likelihood of complications. Treatment begins with pain control, hydration, and bowel rest. In the first 48 to 72 hours of treatment, monitoring is required to prevent morbidity and mortality associated with worsening pancreatitis. When prolonged bowel rest is indicated, enteral nutrition is associated with lower rates of complications, including death, multiorgan failure, local complications, and systemic infections, than parenteral nutrition. In severe cases involving greater than 30% necrosis, antibiotic prophylaxis with imipenem/cilastatin decreases the risk of pancreatic infection. In gallstone-associated pancreatitis, early cholecystectomy and endoscopic retrograde cholangiopancreatography with sphincterotomy can decrease length of hospital stay and complication rates. A multidisciplinary approach to care is essential in cases involving pancreatic necrosis.
The pancreas has endocrine and exocrine functions; the exocrine gland, via the major papilla, releases digestive enzymes into the duodenum via the pancreatic and bile ducts. Premature activation of exocrine enzymes in the pancreas causes inflammation. Acute pancreatitis is the sudden onset of reversible inflammation, whereas chronic pancreatitis is a progressive disorder characterized by ongoing inflammation and destruction that may occur insidiously.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Contrast-enhanced CT is the preferred imaging technique in pancreatitis because of its use in determining disease severity and prognosis. | C | 15 |
| CT should not be ordered routinely for patients with mild acute pancreatitis. | C | 17 |
| Enteral nutrition is preferred over parenteral nutrition for patients with severe pancreatitis, and it is associated with lower complication rates and shorter hospitalizations. | A | 39, 40 |
| Imipenem/cilastatin (Primaxin) use results in a significant decrease in pancreatic infection in patients with pancreatic necrosis; however, prophylactic antibiotics do not reduce mortality. | B | 42 |
| Prophylactic antibiotics should be used only when there is greater than 30% necrosis in the pancreas. | C | 12 |
| In patients with gallstone-associated pancreatitis, cholecystectomy within 48 hours of presentation can shorten the length of hospitalization and does not increase the risk of complications. | B | 45 |
| Severe pancreatitis with infected necrosis or persistent fluid collections should be treated with percutaneous CT-guided aspiration or surgical debridement. | C | 48 |
In 2000, approximately 210,000 adults in the United States were admitted to the hospital for pancreatitis.1 Approximately 50,000 persons are admitted for acute pancreatitis annually, and the number is gradually increasing.2 The most common causes of pancreatitis include gallstones and chronic alcohol use or abuse. Other causes and risk factors are listed in Table 1.3–6

| Choledocholithiasis (40% of cases)3 | |
| Chronic alcohol use or abuse (35% of cases)5 | |
| Endoscopic retrograde cholangiopancreatography (4% of cases)3 | |
| Medication use (2% of cases; e.g., azathioprine [Imuran], didanosine [Videx], estrogens, furosemide [Lasix], pentamidine [Pentam 300], sulfonamides, tetracycline, valproic acid [Depakene])3 | |
| Abdominal trauma (1.5% of cases)3 | |
| Other | |
| Abnormalities of the pancreas (annular pancreas, pancreas divisum, sphincter of Oddi dysfunction)3,4 | |
| Autoimmune disorders3 | |
| Hereditary factors5 | |
| Hypercalcemia (excessive vitamin D therapy, hyperparathyroidism, total parenteral nutrition)3,4 | |
| Hypertriglyceridemia3,4 | |
| Infections (viral, bacterial, fungal, and parasitic)6 | |
| Surgical procedures3 | |
| Toxins (scorpion or snake bites)3 | |
| Tumors4 | |
| Vascular abnormalities (ischemia, vasculitis)3 | |
Using the Atlanta criteria, acute pancreatitis is diagnosed when a patient presents with two of three findings, including abdominal pain suggestive of pancreatitis, serum amylase and/or lipase levels at least three times the normal level, and characteristic findings on imaging.7,8 Acute pancreatitis can vary from mild (mortality rate less than 1%; typically resolves in several days) to severe (mortality rate up to 30%).7 Mortality rates are highest in patients with hemorrhagic pancreatitis, multiorgan dysfunction or failure, and necrotizing pancreatitis.7 In necrotizing pancreatitis, infection or abscess substantially increases the mortality rate. Complications associated with pancreatitis are summarized in Table 2.
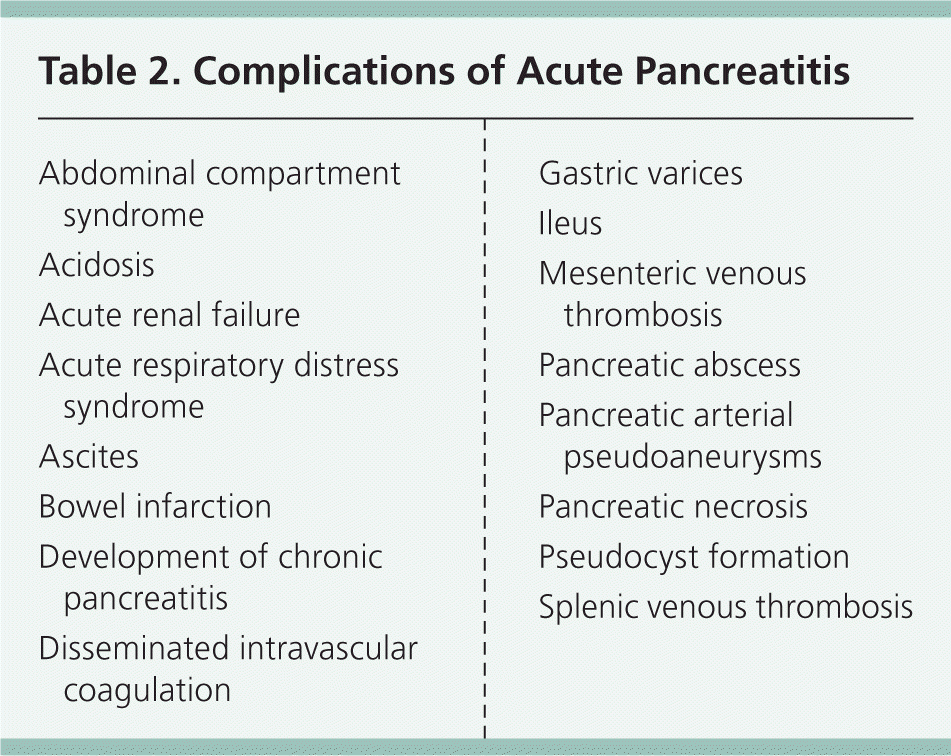
| Abdominal compartment syndrome |
| Acidosis |
| Acute renal failure |
| Acute respiratory distress syndrome |
| Ascites |
| Bowel infarction |
| Development of chronic pancreatitis |
| Disseminated intravascular coagulation |
| Gastric varices |
| Ileus |
| Mesenteric venous thrombosis |
| Pancreatic abscess |
| Pancreatic arterial pseudoaneurysms |
| Pancreatic necrosis |
| Pseudocyst formation |
| Splenic venous thrombosis |
Diagnosis
The differential diagnosis of acute pancreatitis is broad and is summarized in Table 3.
| Acute myocardial infarction |
| Cholangitis |
| Cholecystitis |
| Diabetic ketoacidosis |
| Gastric outlet obstruction |
| Gastric volvulus |
| Hepatitis |
| Intestinal infarction |
| Pancreatic cancer |
| Perforated peptic ulcer |
| Tubo-ovarian abscess |
HISTORY AND PHYSICAL EXAMINATION
Patients with pancreatitis commonly present with sudden onset of abdominal pain in the left upper quadrant, periumbilical region, and/or epigastrium, although in some cases acute pancreatitis may be painless. Initially, the pain worsens after eating or drinking, especially fatty foods, and then typically becomes constant over time. The pain may radiate throughout the abdomen and into the chest or mid back, is often associated with nausea and vomiting, and may be worse when the patient is supine. Patients may also report indigestion, abdominal fullness, distension, clay-colored stools, decreased urine output, and frequent hiccups. Occasionally, patients may present with syncope or subjective fever.
The physical examination findings may be normal or reveal fever, hypotension, tachycardia, tachypnea, or diaphoresis. Abdominal examination typically reveals notable tenderness to palpation, guarding, and possible signs of peritoneal irritation, distension, or rigidity. Bowel sounds are typically decreased. Jaundice may be present. In severe disease, patients may present with altered mental status. Overall, history and physical examination have moderate accuracy, especially when findings are abnormal (positive likelihood ratio [LR+] = 3.2; negative likelihood ratio [LR–] = 0.8).9 Two physical signs associated with pancreatitis are Cullen sign (ecchymosis and edema in the subcutaneous tissue around the umbilicus) and Grey Turner sign (ecchymosis of the flank).
LABORATORY TESTS
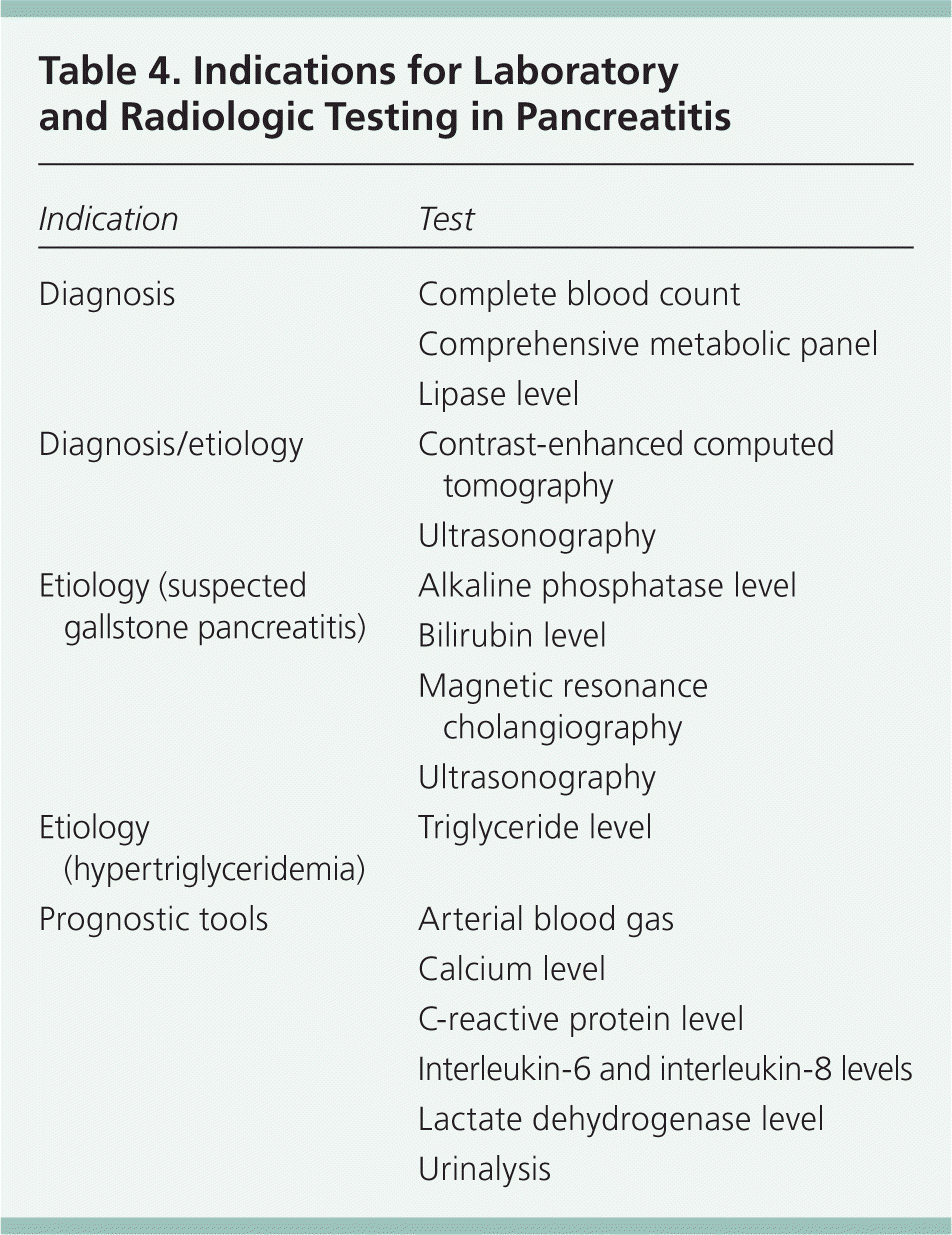
Laboratory testing can assist in diagnosis, classify the severity of disease, and predict outcomes (Table 4). Specific tests that should be ordered at presentation include a complete blood count; a comprehensive metabolic panel including renal and hepatic function; urinalysis; and measurement of lipase, calcium, lactate dehydrogenase, and triglyceride levels. If alcohol abuse is a factor, magnesium and phosphorous levels should be assessed. Depending on the clinical scenario, measurement of arterial blood gases, C-reactive protein level, or interleukin-6 (IL-6)/interleukin-8 (IL-8) levels can be helpful in determining severity of disease and prognosis. Although IL-6 and IL-8 levels may be helpful prognostically as part of the BALI score (Table 510 ), their lack of rapid availability limits their use.
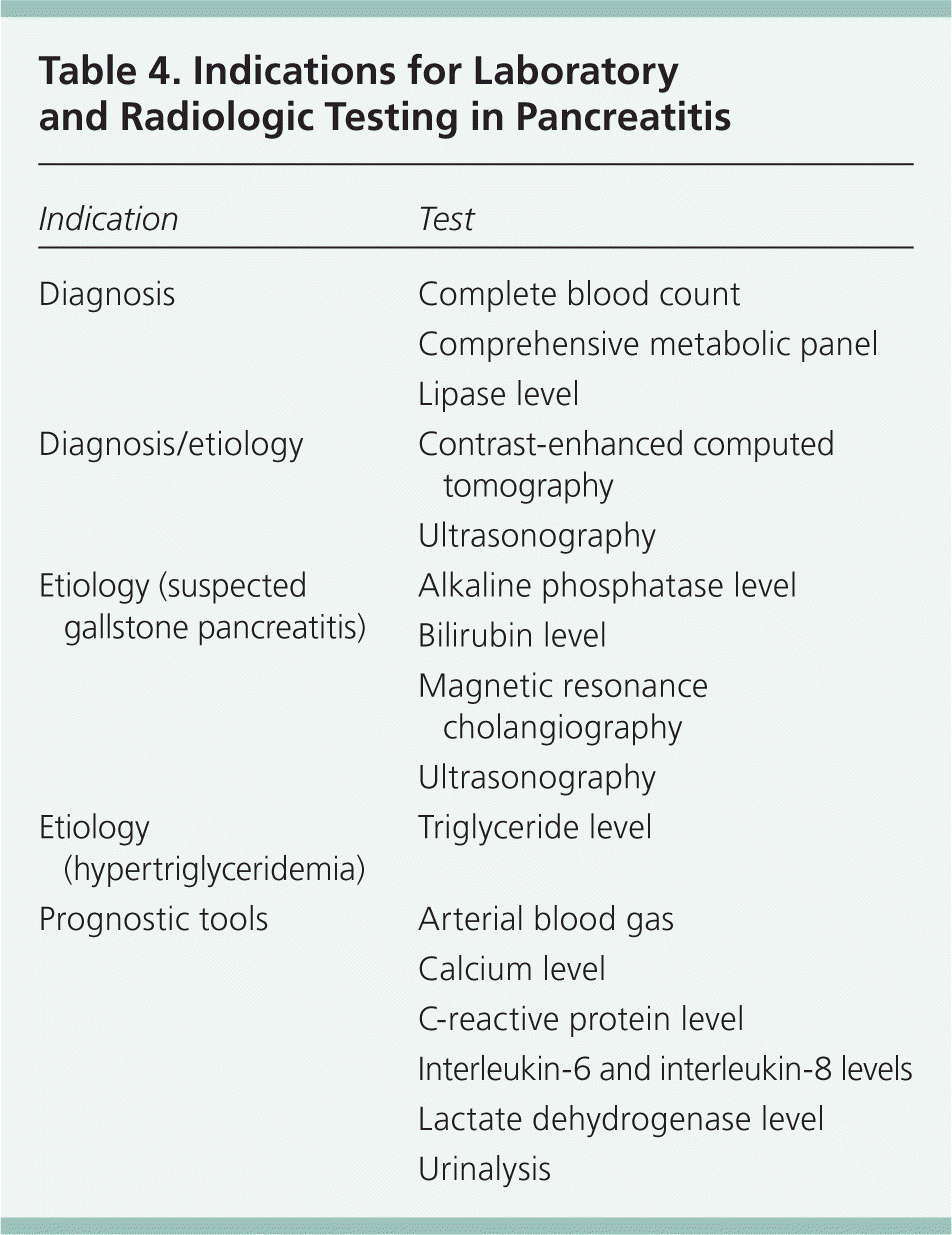
| Indication | Test |
|---|---|
|
|
|
|
|
|
|
|
|
|
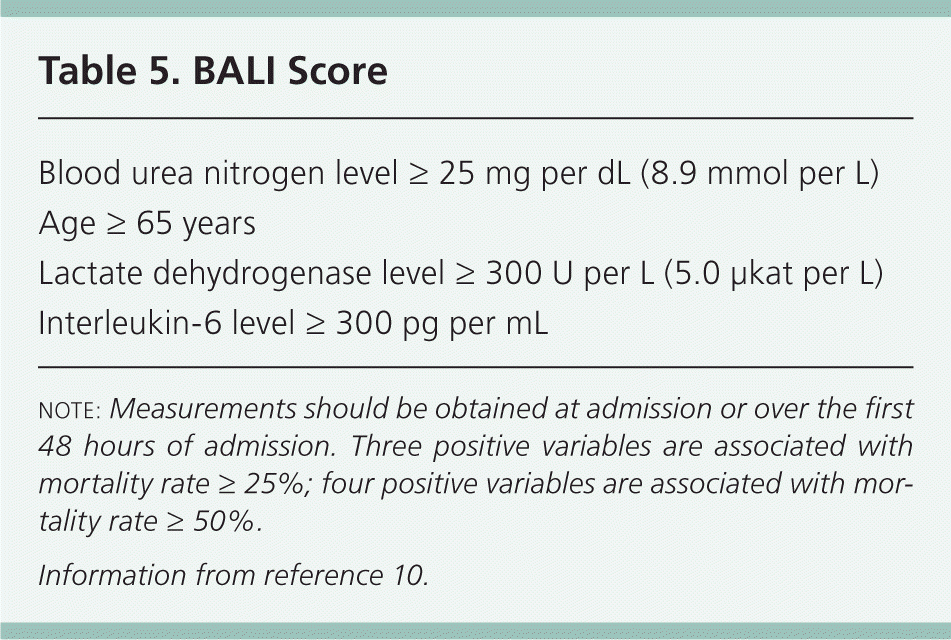
| Blood urea nitrogen level ≥ 25 mg per dL (8.9 mmol per L) |
| Age ≥ 65 years |
| Lactate dehydrogenase level ≥ 300 U per L (5.0 μkat per L) |
| Interleukin-6 level ≥ 300 pg per mL |
Lipase level testing is more sensitive and specific than measuring amylase levels, because amylase is also produced by the salivary glands and levels may be normal in patients with recurrent alcoholic pancreatitis.11 Lipase or amylase levels greater than three times the normal amount are considered diagnostic for pancreatitis.12 In addition, a lipase-to-amylase ratio of greater than 4 (LR+ = 7.3) or 5 (LR+ = 31.0) strongly supports an alcoholic cause of pancreatitis.13 Urinary trypsinogen activation peptide has been used to predict the severity of pancreatitis. Its accuracy is similar to that of other available markers and prognostic tools.14
IMAGING STUDIES
There have been considerable advances in imaging modalities for the evaluation of abdominal pain. Although this has potential to aid in diagnosis and management of pancreatitis, inappropriate imaging can increase cost, radiation exposure, and complication rates without significant benefit to patients. The American College of Radiology and the American College of Gastroenterology (ACG) have developed evidence-based guidelines to assist in determining appropriate radiologic testing for a given clinical situation.8,15
Current ACG guidelines recommend that all patients with acute pancreatitis be evaluated using abdominal ultrasonography.8 Ultrasonography may be helpful in diagnosing gallstone-associated pancreatitis; however, it is somewhat limited in prognostication and when overlying bowel gas is present or gallstones are in the distal bile duct.16
Contrast-enhanced computed tomography (CT) is considered the diagnostic standard for radiologic evaluation of acute pancreatitis because it has demonstrated success in the prediction of disease severity and prognosis.16 It is considered the modality of choice for patients with severe abdominal pain and when necrotic pancreatitis or other complications are clinically suspected. However, if it is not clinically indicated (e.g., in a stable patient with mild pancreatitis), CT should not be performed for the sole purpose of assessing severity of disease at admission.17
Nonenhanced magnetic resonance imaging has a sensitivity of 79% and a specificity of 92% compared with CT for the identification of severe pancreatitis.18 It is particularly helpful in patients for whom intravenous contrast media is contraindicated. Additionally, it may offer advantages over CT for evaluation of the pancreatic duct and pancreatitis-associated fluid collections.15 In general, the use of magnetic resonance imaging should be limited to patients with an unclear diagnosis or patients who do not demonstrate clinical improvement within two to three days of admission to the hospital.8
Endoscopic ultrasonography can be useful in diagnosing choledocholithiasis as the etiology19 and in determining which patients may benefit from endoscopic retrograde cholangiopancreatography (ERCP).20 Magnetic resonance cholangiopancreatography is a noninvasive imaging method of detecting choledocholithiasis that does not require gadolinium and has similar usefulness to ERCP.21 Table 6 summarizes the accuracy of diagnostic tests for acute pancreatitis.18,22,23
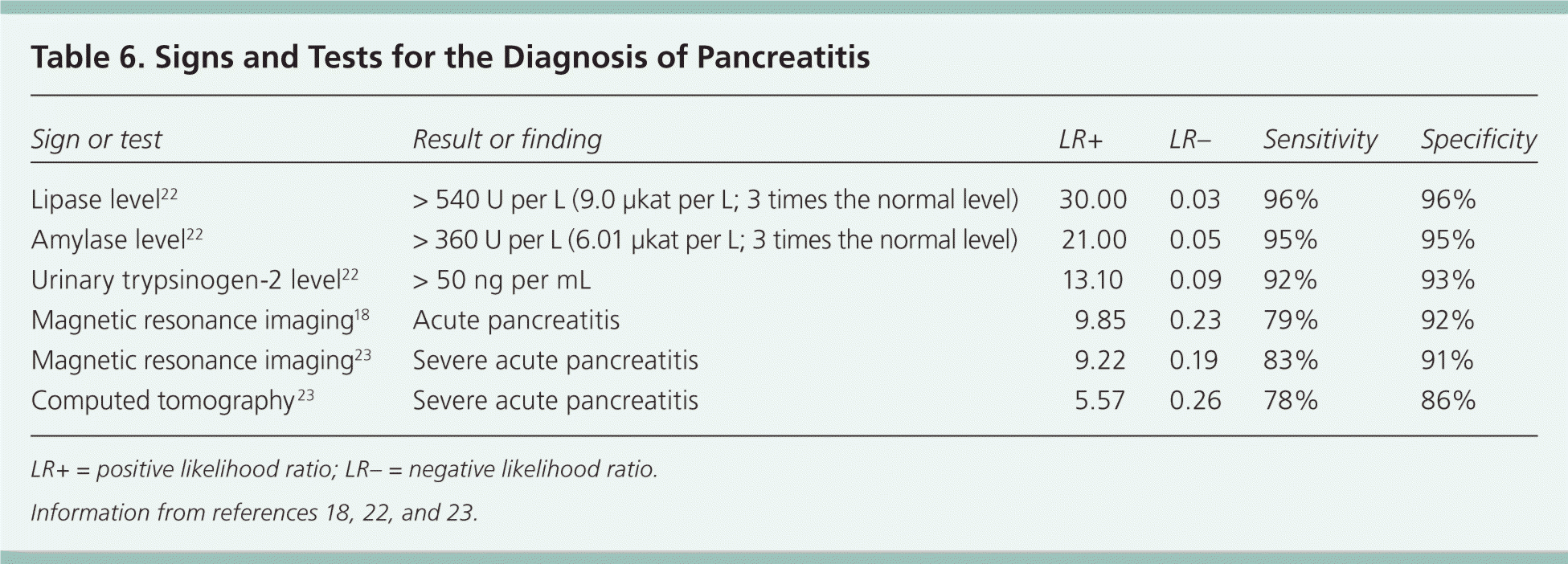
| Sign or test | Result or finding | LR+ | LR– | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Lipase level22 | > 540 U per L (9.0 μkat per L; 3 times the normal level) | 30.00 | 0.03 | 96% | 96% |
| Amylase level22 | > 360 U per L (6.01 μkat per L; 3 times the normal level) | 21.00 | 0.05 | 95% | 95% |
| Urinary trypsinogen-2 level22 | > 50 ng per mL | 13.10 | 0.09 | 92% | 93% |
| Magnetic resonance imaging18 | Acute pancreatitis | 9.85 | 0.23 | 79% | 92% |
| Magnetic resonance imaging23 | Severe acute pancreatitis | 9.22 | 0.19 | 83% | 91% |
| Computed tomography23 | Severe acute pancreatitis | 5.57 | 0.26 | 78% | 86% |
Assessment of Severity and Prognosis
Tools have been developed to predict the severity of pancreatitis and the likelihood of complications and mortality. They have been shown to be superior to clinical judgment alone, and should be used in conjunction with typical clinical criteria, such as presence of comorbid conditions, age, and first episode of pancreatitis.
The Atlanta criteria use early prognostic signs, organ failure, and local complications to define disease severity24,25 (Table 725 ). Early prognostic signs include a Ranson score of 3 or greater, or an acute physiology and chronic health evaluation (APACHE II) score of 8 or greater. Organ failure is defined as shock, hypoxemia (partial arterial oxygen tension of 60 mm Hg or less), creatinine level greater than 2 mg per dL (177 μmol per L), or gastrointestinal bleeding (greater than 500 mL per 24 hours). Local complications include necrosis, abscess, or pseudocyst.24,26
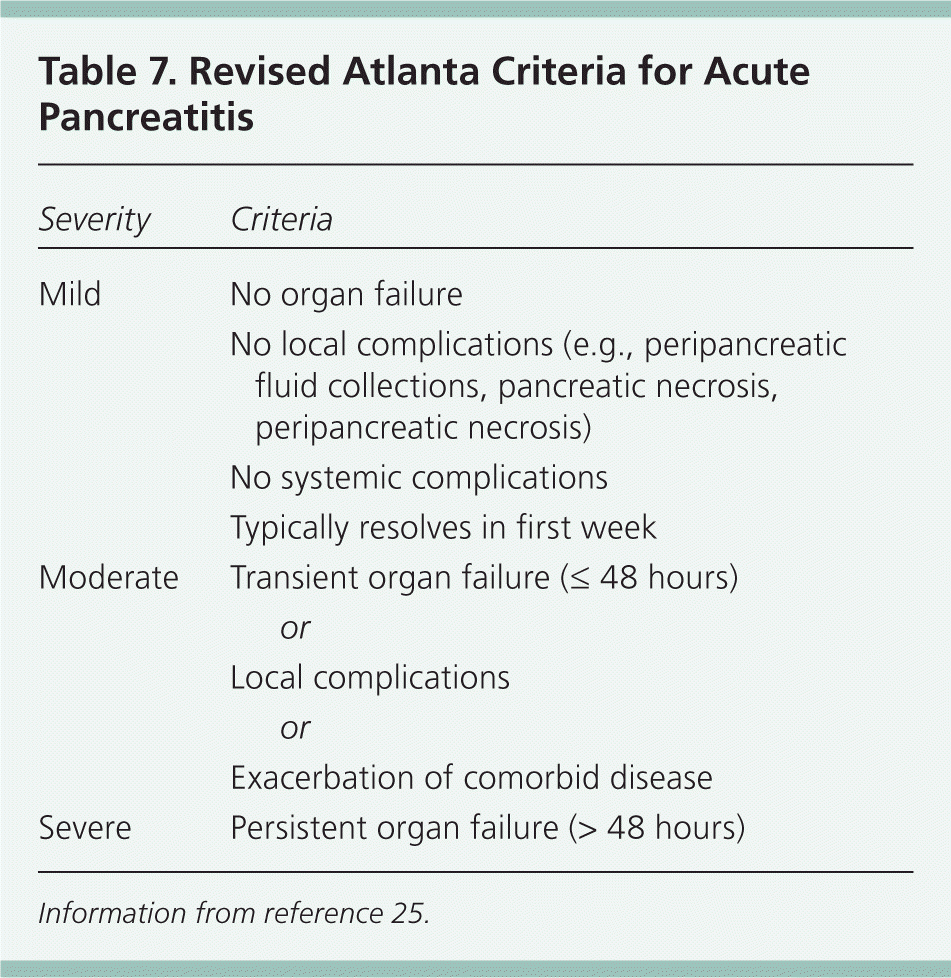
| Severity | Criteria | |
|---|---|---|
|
| |
|
| |
|
| |
The Ranson score evaluates 11 factors within 48 hours of hospital admission to predict severity of pancreatitis and risk of mortality. However, the sensitivity for predicting poor outcome is only 70%.27 The APACHE II scoring system uses 12 criteria to predict the severity of pancreatitis, with the risk of death increasing as the score increases.28 It has been shown to have a sensitivity of up to 95% when used daily for reassessment of patients in the intensive care unit,29,30 although other study results have demonstrated notably lower sensitivity.31
Newer risk scores include the Modified Glasgow (Imrie) prediction score, the bedside index of severity in pancreatitis, the BALI score, and the CT severity index. In a comparison of nine clinical and radiologic prognostic tools, none was demonstrated to be superior to the others by a statistically significant level.17
An advantage of the BALI score (Table 5) is simplicity because it evaluates only four variables: blood urea nitrogen level, age, lactate dehydrogenase level, and IL-6 level.10 Measurements are taken at admission and can be repeated throughout the first 48 hours of hospitalization. A score of 3 is associated with a mortality rate ≥ 25%, and a score of 4 is associated with a mortality rate ≥ 50%. These results were similar to the predictive abilities of the Ranson, Glasgow, and APACHE II predictive models.10 Given the ease of use of the BALI score as a prognostic tool, it should be considered if IL-6 levels are easily obtained.
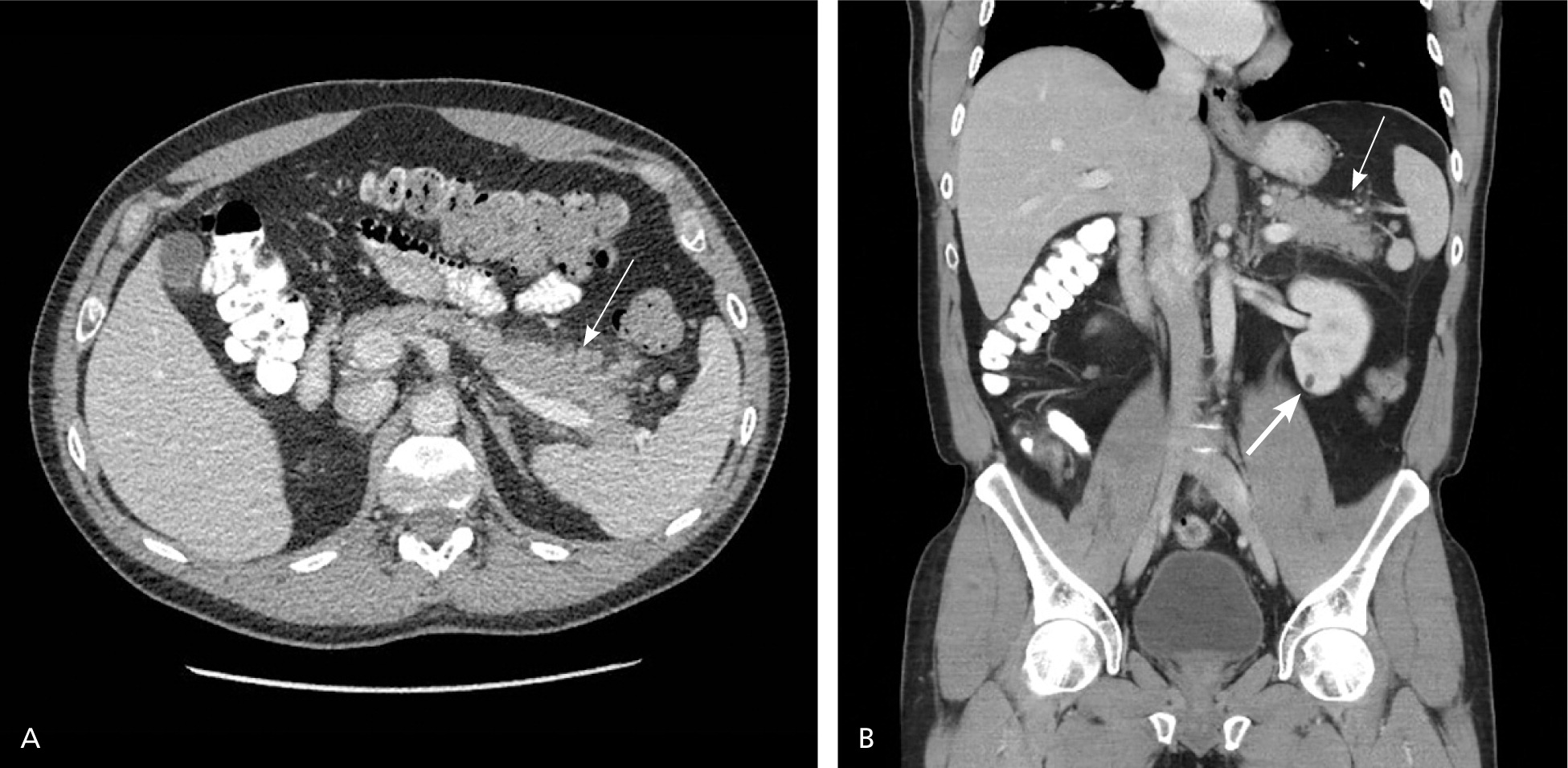
The CT severity index is based on CT findings at admission and evaluates for the presence of peripancreatic inflammation (Figure 1), phlegmon, and, if present, the amount of pancreatic necrosis (Figure 2). The index is summarized in Table 8.32–34 Points for CT grade are combined with points from the necrosis score. A total score of 5 or greater is associated with a statistically significant increase in morbidity and mortality.34 Several studies have found that CT severity index is superior to Ranson and APACHE II in predicting severity and outcomes in pancreatitis, with a sensitivity of 87% and specificity of 83% (LR+ = 5.1; LR– = 0.16).17,34–36
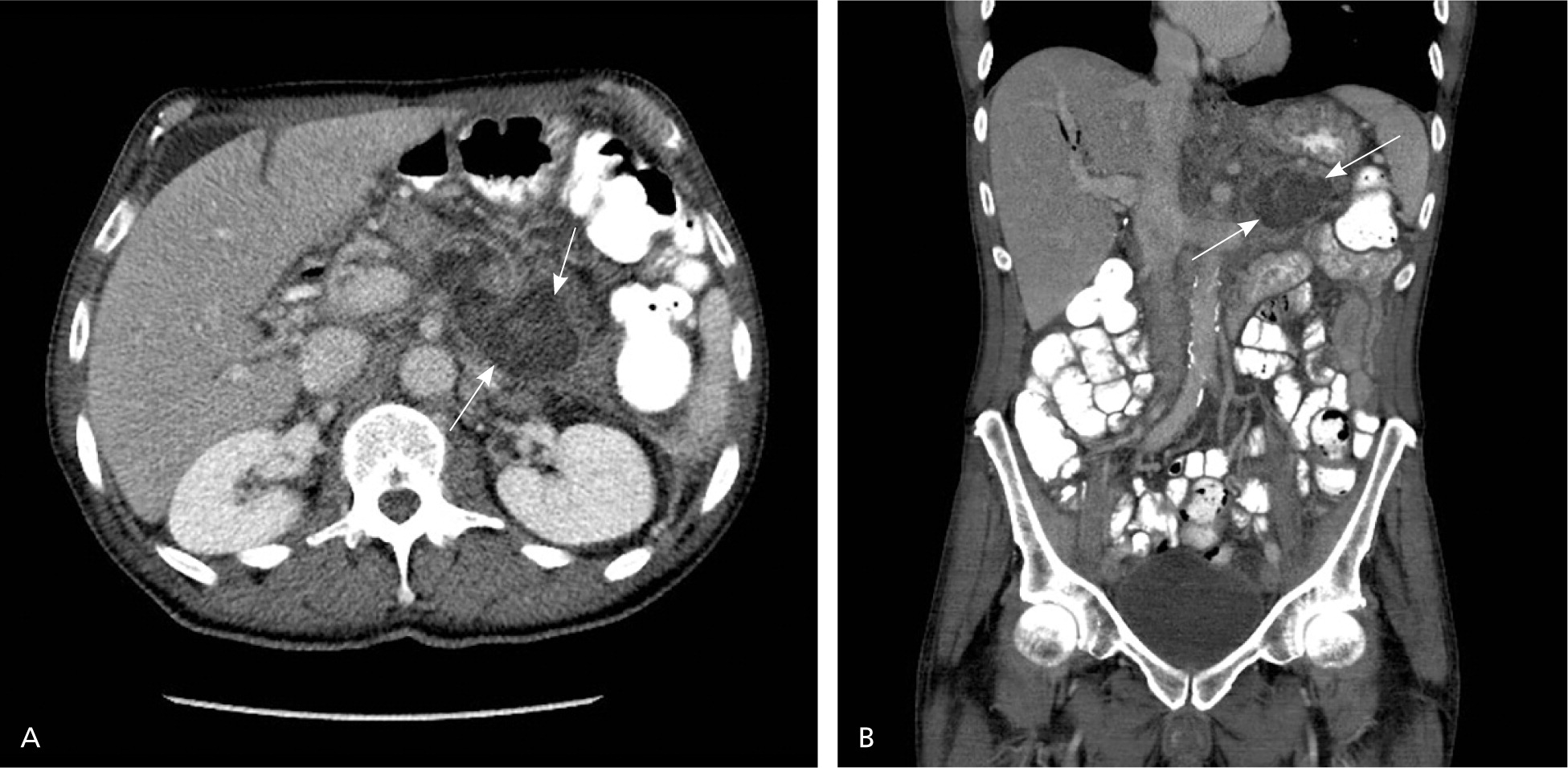
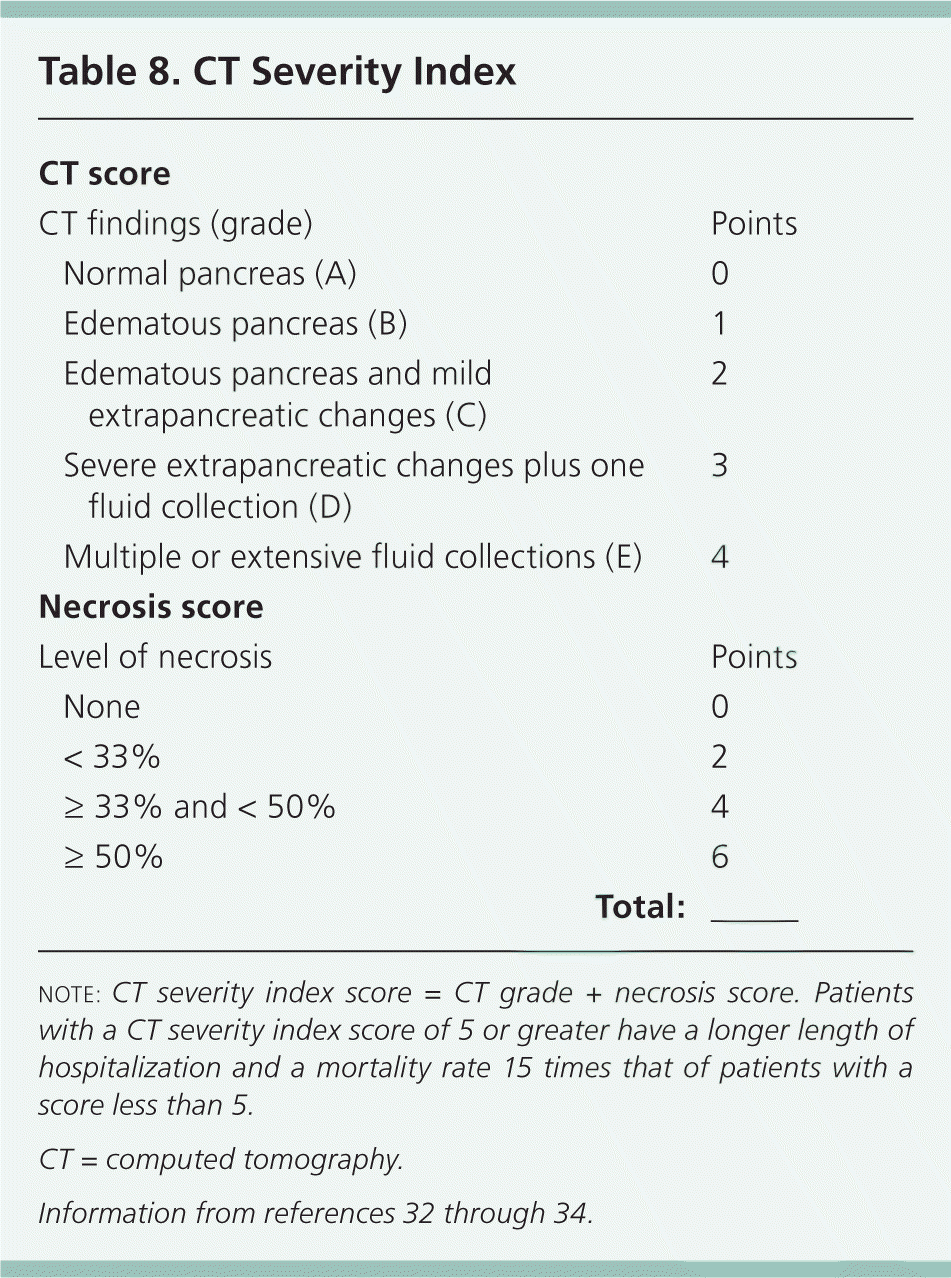
| CT score | |||
| CT findings (grade) | Points | ||
| Normal pancreas (A) | 0 | ||
| Edematous pancreas (B) | 1 | ||
| Edematous pancreas and mild extrapancreatic changes (C) | 2 | ||
| Severe extrapancreatic changes plus one fluid collection (D) | 3 | ||
| Multiple or extensive fluid collections (E) | 4 | ||
| Necrosis score | |||
| Level of necrosis | Points | ||
| None | 0 | ||
| < 33% | 2 | ||
| ≥ 33% and < 50% | 4 | ||
| ≥ 50% | 6 | ||
| Total:___ | |||
Treatment
NONSURGICAL TREATMENT
Pancreatitis is treated with bowel rest, fluid hydration, and pain control. Patients with mild pancreatitis may be treated as outpatients; however, most patients require hospitalization. In outpatients, nutrition and hydration should be maintained via clear fluids, and pain control should be managed with oral narcotics.
Hospitalized patients should be placed on bowel rest and receive fluid resuscitation. Initially, 20 mL per kg of lactated Ringer solution or normal saline should be administered over 60 to 90 minutes, followed by 250 to 500 mL per hour for the next 48 hours to maintain a urine output of 0.5 mL per kg per hour and with a goal of decreasing the blood urea nitrogen level.8,37,38 Typically, patients can be transitioned to oral clear liquids when pain is well controlled (e.g., no narcotics required), and subsequently transitioned to low-fat full liquids and then a low-fat regular diet.
During the first 48 to 72 hours of treatment, patients should be followed for worsening disease. Initially, blood pressure, pulse, oxygen saturation, and urine output should be monitored frequently (every one to two hours). Hypotension, hypoxemia, or oliguria that is unresponsive to intravenous hydration should prompt transfer to the intensive care unit. The physical examination should be repeated every four to eight hours after presentation, with attention to the presence of altered mental status or marked firmness of the abdomen, which suggests abdominal compartment syndrome or third spacing of fluid.
A comprehensive metabolic panel; complete blood count; and calcium, magnesium, serum glucose, and blood urea nitrogen levels should be obtained every six to 12 hours, depending on the patient's status. Hypocalcemia and hypomagnesemia should be corrected intravenously. Likewise, elevated glucose levels should be managed with insulin. Persistent hemoconcentration or an elevated blood urea nitrogen level may indicate inadequate hydration or renal injury, and increased intravenous fluids should be considered. CT may be repeated if there is a poor response to standard therapy to assess for complications or worsening pancreatitis.
Traditionally, patients who require prolonged bowel rest have been provided parenteral nutrition. However, a meta-analysis demonstrated that nasojejunal nutrition results in fewer infections (relative risk = 0.45; number needed to treat = 7; 95% confidence interval [CI], 5 to 16), decreases surgical interventions (relative risk = 0.48; 95% CI, 0.23 to 0.99), and leads to shorter hospital stays without change in complication or mortality rates, compared with parenteral nutrition.8,39 A 2010 Cochrane review found that patients treated with enteral nutrition had lower rates of complications, a decreased requirement for operative treatment, and a nonsignificant trend toward shorter hospital stays.40 Nasogastric and nasojejunal nutrition have similar safety and effectiveness profiles.8 Some patients, in particular those with profound ileus or very low oncotic pressure, may not tolerate enteral feeds.
Approximately one-third of patients with necrotic pancreatitis develop infections.26 There has been debate about the benefit of prophylactic antibiotic treatment in these patients. Although an underpowered meta-analysis of six trials demonstrated no statistically significant difference in mortality or infected necrosis between treated and untreated patients, the trend in both cases favored prophylaxis over no prophylaxis (mortality rate of 10% vs. 17%, respectively; infection rate of 20% vs. 29%, respectively).41 A subsequent Cochrane review concluded that there was no difference between prophylaxis and nonprophylaxis with respect to mortality, infected pancreatic necrosis, non-pancreatic and overall infections, operative treatment, and fungal infection rates; however, none of the studies was adequately powered. It did conclude, however, that imipenem/cilastatin (Primaxin) as monotherapy resulted in a statistically significant decrease in pancreatic infection.42 The American Gastroenterological Association guidelines recommend restricting the use of prophylactic antibiotics to patients with necrosis involving greater than 30% of the pancreas, whereas the ACG recommends routine prophylaxis for patients with extrapancreatic infection but not those with severe acute pancreatitis or sterile necrosis.8,12
The Dutch Acute Pancreatitis Study Group, evaluating the use of a probiotic cocktail for the prevention of infectious complications, found a statistically significant increase in deaths in the group treated with the probiotic cocktail (number needed to harm = 11; 95% CI, 6 to 43).43 Probiotic use in acute pancreatitis is therefore contraindicated.
In China, herbal medicines, including licorice root, ginger root, ginseng, peony root, and cinnamon Chinese bark, are used for the treatment of pancreatitis. A Cochrane review evaluating 15 Chinese studies determined that the use of herbal medicine appears to reduce the rates of mortality, surgical intervention, multiorgan failure, and systemic infection; however, the quality of the studies was low.44 Furthermore, the herbs may not be readily available in the United States.
SURGICAL TREATMENT
In patients with gallstone-associated pancreatitis, cholecystectomy within 48 hours of presentation can shorten the patient's length of stay in the hospital when compared with cholecystectomy after resolution of pain and a trend toward normal enzyme levels (3.5 vs. 5.8 days; P = .0016).45 Additionally, early cholecystectomy does not increase the risk of complications secondary to surgery.45 However, cholecystectomy should not be performed in patients with necrotizing acute pancreatitis until inflammation has decreased and fluid collections are no longer increasing in size.8
ERCP with sphincterotomy may decrease mortality (5.2% vs. 9.1%; number needed to treat = 26) and complications (25% vs. 38%; number needed to treat = 7.6) compared with no sphincterotomy in patients with gallstone-associated pancreatitis.46 A Cochrane review found a statistically significant decrease in complication rates only in severe gallstone pancreatitis; there were nonsignificant decreases in mortality rates for mild and severe gallstone pancreatitis as well as complication rates in mild gallstone pancreatitis.47 As a result, the ACG guidelines recommend limiting ERCP to patients with acute pancreatitis complicated by acute cholangitis and patients with gallstone-associated pancreatitis who demonstrate laboratory or clinical evidence of unresolved biliary obstruction.8
Finally, in patients with asymptomatic fluid collections or necrosis, no immediate intervention is required.8 However, in patients with severe pancreatitis and infected necrosis or persistent fluid collections, percutaneous CT-guided aspiration or surgical debridement (necrosectomy) is required.48 In these patients, a minimally invasive approach is preferred to an open approach.8 Collected fluid should be examined by Gram stain and cultured to determine the most appropriate antibiotic.8 These patients need close collaboration between interventional radiology, interventional endoscopy/gastroenterology, and pancreatic surgeons.
Data Sources: The primary literature search was completed using Essential Evidence Plus and included searches of the Cochrane database and National Guideline Clearinghouse with the term pancreatitis. In addition, a PubMed search was completed using the terms pancreatitis and acute pancreatitis. Additional resources were identified through review of references cited in American Gastroenterological Association and American College of Radiology guidelines. Search dates: November 7, 2012, and June 13, 2014.