A more recent article on abdominal aortic aneurysm is available.
Am Fam Physician. 2015;91(8):538-543
Related editorial: Pitfalls of Direct-to-Consumer Vascular Screening Tests.
Related Putting Prevention into Practice: Screening for Abdominal Aortic Aneurysm, and U.S. Preventive Services Task Force Recommendation Statement: Screening for Abdominal Aortic Aneurysm: Recommendation Statement
Author disclosure: No relevant financial affiliations.
Abdominal aortic aneurysm refers to abdominal aortic dilation of 3.0 cm or greater. The main risk factors are age older than 65 years, male sex, and smoking history. Other risk factors include a family history of abdominal aortic aneurysm, coronary artery disease, hypertension, peripheral artery disease, and previous myocardial infarction. Diagnosis may be made by physical examination, an incidental finding on imaging, or ultrasonography. The U.S. Preventive Services Task Force released updated recommendations for abdominal aortic aneurysm screening in 2014. Men 65 to 75 years of age with a history of smoking should undergo one-time screening with ultrasonography based on evidence that screening will improve abdominal aortic aneurysm–related mortality in this population. Men in this age group without a history of smoking may benefit if they have other risk factors (e.g., family history of abdominal aortic aneurysm, other vascular aneurysms, coronary artery disease). There is inconclusive evidence to recommend screening for abdominal aortic aneurysm in women 65 to 75 years of age with a smoking history. Women without a smoking history should not undergo screening because the harms likely outweigh the benefits. Persons who have a stable abdominal aortic aneurysm should undergo regular surveillance or operative intervention depending on aneurysm size. Surgical intervention by open or endovascular repair is the primary option and is typically reserved for aneurysms 5.5 cm in diameter or greater. There are limited options for medical treatment beyond risk factor modification. Ruptured abdominal aortic aneurysm is a medical emergency presenting with hypotension, shooting abdominal or back pain, and a pulsatile abdominal mass. It is associated with high prehospitalization mortality. Emergent surgical intervention is indicated for a rupture but has a high operative mortality rate.
Abdominal aortic aneurysm (AAA) is an abdominal aortic dilation of 3.0 cm or greater.1 The prevalence of AAA increases with age. It is uncommon in persons younger than 50 years; however, 12.5% of men and 5.2% of women 74 to 84 years of age have AAA.1 It accounts for approximately 11,000 deaths each year in the United States, with mortality rates from ruptured AAAs reaching up to 90%.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| One-time screening for AAA with ultrasonography should be performed in men 65 to 75 years of age who have smoked 100 cigarettes or more in their lifetime. | B | 4, 9 |
| One-time screening for AAA with ultrasonography should be selectively offered in men 65 to 75 years of age who have never smoked, but have risk factors for AAA. | B | 9 |
| Current evidence is insufficient to recommend for or against AAA screening in women 65 to 75 years of age who have smoked 100 cigarettes or more in their lifetime. | B | 9 |
| AAA screening should not be performed in women who have never smoked. | B | 9 |
| Patients with AAAs 3.0 to 3.9 cm in diameter should be monitored with ultrasonography every two to three years. | C | 1 |
| Patients with AAAs 4.0 to 5.4 cm in diameter should be monitored with ultrasonography or computed tomography every six to 12 months. | C | 1 |
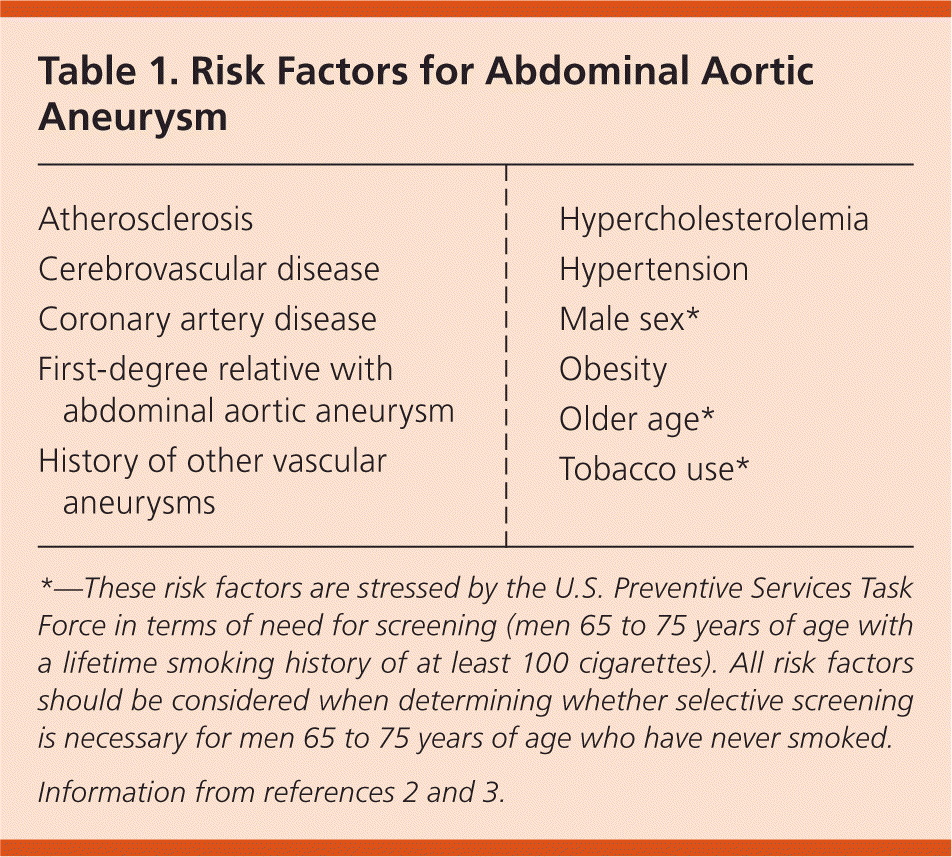
Aneurysms develop as a result of degeneration of the arterial media and elastic tissues.1 Risk factors for AAA are similar to those of other cardiovascular diseases. The key risk factors are male sex, smoking, age older than 65 years, coronary artery disease, hypertension, previous myocardial infarction, peripheral arterial disease, and a family history of AAA1,3,4 (Table 12,3). Blacks appear to be at lower risk.4
Beyond the inherent risk of rupture, patients with AAA are also at an increased risk of cardiovascular disease and death independent of other factors.5 The degree to which risk factors impact AAA vs. atherosclerosis varies. For example, dyslipidemia is an important coronary artery disease risk factor, although its role in AAA remains uncertain, and diabetes mellitus may have a negative association with AAA.2,4
Presentation
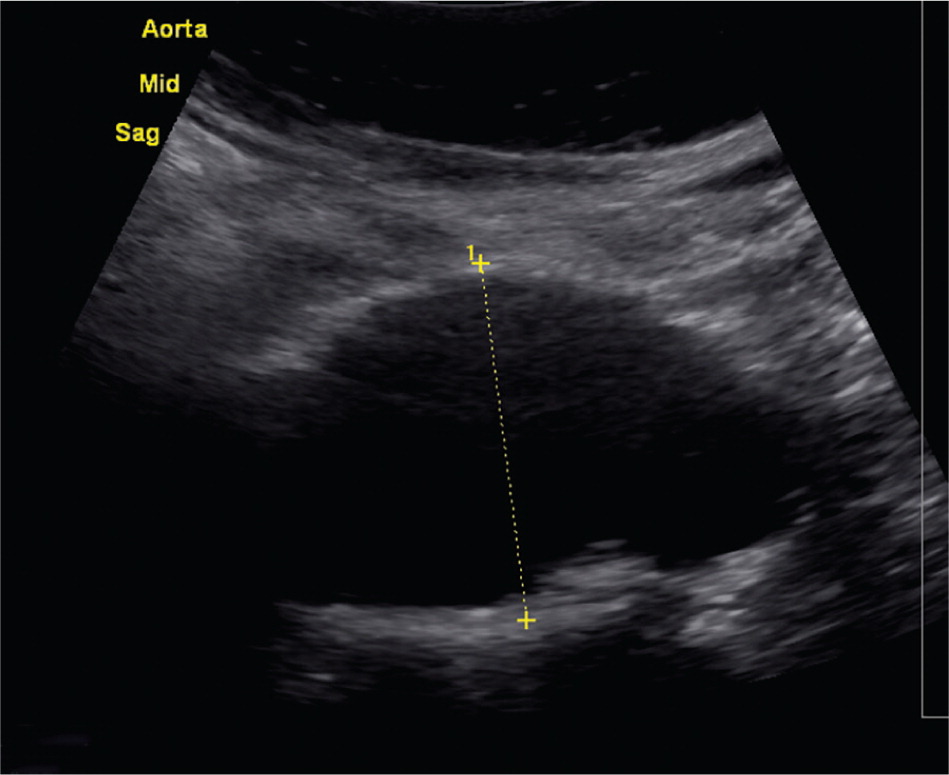
Physical examination with abdominal palpation is only moderately sensitive for the detection of AAA, with one study demonstrating a sensitivity of 68% and specificity of 75%.6 The most common finding is palpation of a pulsatile mass around the level of the umbilicus. Abdominal auscultation may reveal the presence of a bruit. The accuracy of abdominal palpation is reduced by obesity, abdominal distention, and smaller aneurysm size. In particular, abdominal girth greater than 100 cm (39.4 in) is associated with decreased sensitivity for identification with palpation.6 An aneurysm may rarely produce findings related to compression of adjacent structures, such as lower extremity edema related to compression of the inferior vena cava.7
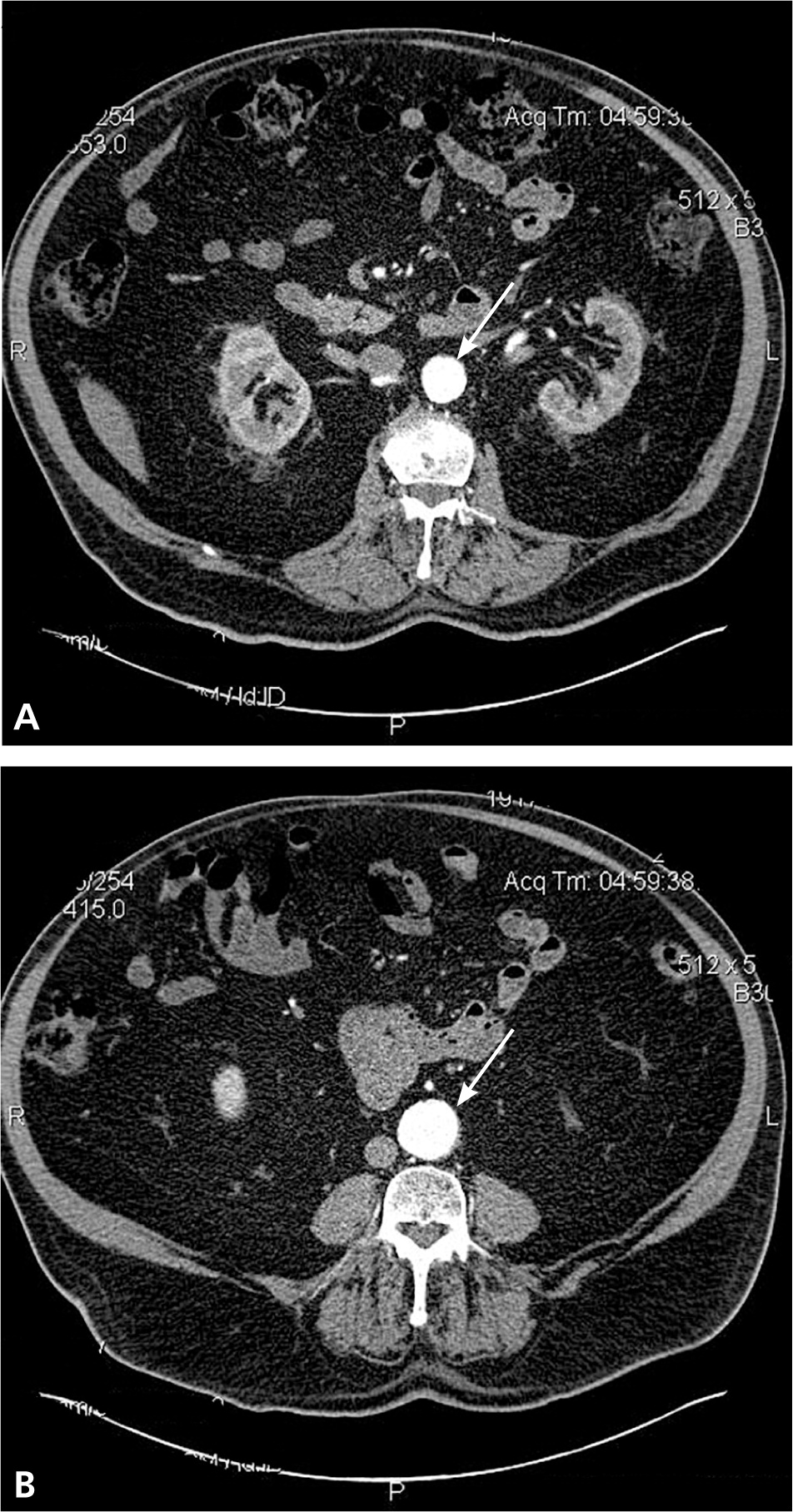
A ruptured AAA is a medical emergency associated with high mortality rates. The classic syndrome is characterized by hypotension, shooting abdominal or back pain, and a pulsatile abdominal mass. This triad may be incomplete or absent, and misdiagnosis can occur in up to 60% of cases. Therefore, physicians must be mindful of atypical presentations and attentive to new-onset, nonspecific back or abdominal pain in patients at risk of AAA.8
Screening
Because AAA is most often clinically silent, screening can improve detection. Ultrasonography has a high sensitivity and specificity (95% and nearly 100%, respectively) for detecting AAA when performed in a setting experienced in the use of ultrasonography.4,9 Additionally, there are no significant harms associated with abdominal ultrasonography.4 Although larger studies are needed, preliminary data suggest that family physicians can be trained to successfully screen for AAA in the office setting.10
Four randomized, controlled, population-based studies provide much of the available data on AAA screening.11–14 The Multicentre Aneurysm Screening Study was the largest, following approximately 70,000 men between 65 and 74 years of age for 10 years.11 Participants were randomized to an offer of ultrasonography or to a control group. Those with AAA detected at screening were followed by ultrasound surveillance or elective surgery based on predefined criteria. The reduction in AAA-related mortality improved from 42% at four-year follow-up to 48% at 10-year follow-up, demonstrating continued benefit over the duration of the study.11 This program also demonstrated continued cost-effectiveness, particularly as the study progressed, because the major costs of screening occur early with initial screening and intervention.15 Other data have substantiated the cost-effectiveness of AAA screening.16
As studies such as the Multicentre Aneurysm Screening Study indicate, the main benefit of screening is decreased AAA-related mortality.15 However, this does not translate to improved all-cause mortality in men or women.17 Persons with the greatest potential benefit from screening have the major risk factors of male sex, increased age, and history of smoking. Approximately 238 men older than 65 years need to be screened to prevent one AAA-related death.18,19 Men younger than 65 years and those who have never smoked have a lower risk of developing AAA.9 In addition, women are at lower risk of developing AAA. Available mortality data have not demonstrated significant benefit from screening women.4,18 Family history of AAA may be an important screening consideration because it doubles the risk, and some recommendations include this as a consideration for men and women.20
The risks of screening include the morbidity and mortality associated with elective repair. For example, open repair has a mortality rate of 4.2% and a complication rate of 32%.4 However, this risk is smaller than that of AAA-related mortality in the absence of screening. Other risks include a transient increase in anxiety and lower self-rated health scores among individuals being screened. These differences resolve within six weeks after screening.4
In 2014, the U.S. Preventive Services Task Force (USPSTF) updated its 2005 guideline on ultrasonography screening for AAA. The USPSTF continues to recommend one-time screening with ultrasonography for men 65 to 75 years of age with a history of smoking (level B recommendation).9 Of note, a history of smoking is defined as at least 100 cigarettes over the individual's lifetime. The USPSTF recommends that clinicians selectively offer screening in men 65 to 75 years of age who have never smoked (level C recommendation). Risk factors associated with a higher likelihood of AAA include first-degree relatives with AAA, history of other vascular aneurysms, coronary artery disease, cerebrovascular disease, atherosclerosis, hypercholesterolemia, obesity, and hypertension (Table 12,3). Factors associated with a decreased risk of AAA include black race, Hispanic ethnicity, and diabetes. Of note, the perceived net benefit to screening this population is thought to be small.9
The main difference between the 2005 and 2014 guidelines involves screening in women. In 2005, the guideline recommended against screening in all women. The 2014 guideline has been updated to suggest that the benefit of screening in women 65 to 75 years of age with a history of smoking is inconclusive (level I statement). The USPSTF continues to recommend against screening in women 65 to 75 years of age who have never smoked (level D recommendation). Although men and women 74 to 84 years of age have increased risk of AAA, this group is less likely to benefit from screening and subsequent surgery because of competing comorbidities.1,9
Surveillance
The natural history of AAA shows that as aneurysms increase in size, they expand at a greater rate (Table 21) and the risk of rupture increases (Table 31,2). Therefore, in persons found to have aneurysms on initial screening, regular surveillance is needed every six months to three years, depending on aneurysm size. A meta-analysis found that each 0.5-cm increase in diameter increases the growth rate by 0.6 mm per year; however, this study also suggests that overall growth rates and risk of rupture are somewhat less than previously suggested and may support longer intervals for surveillance21 (Table 41,21,22).
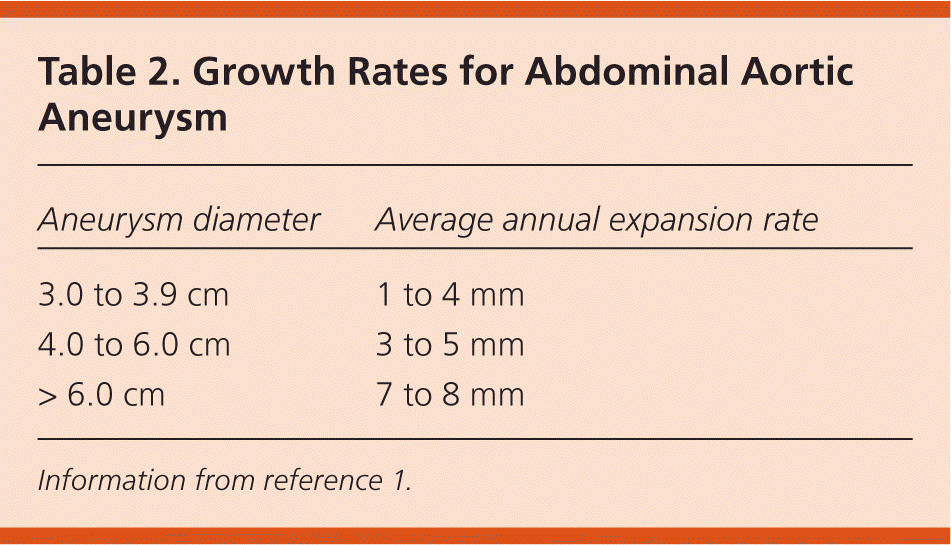
| Aneurysm diameter | Average annual expansion rate |
|---|---|
| 3.0 to 3.9 cm | 1 to 4 mm |
| 4.0 to 6.0 cm | 3 to 5 mm |
| > 6.0 cm | 7 to 8 mm |
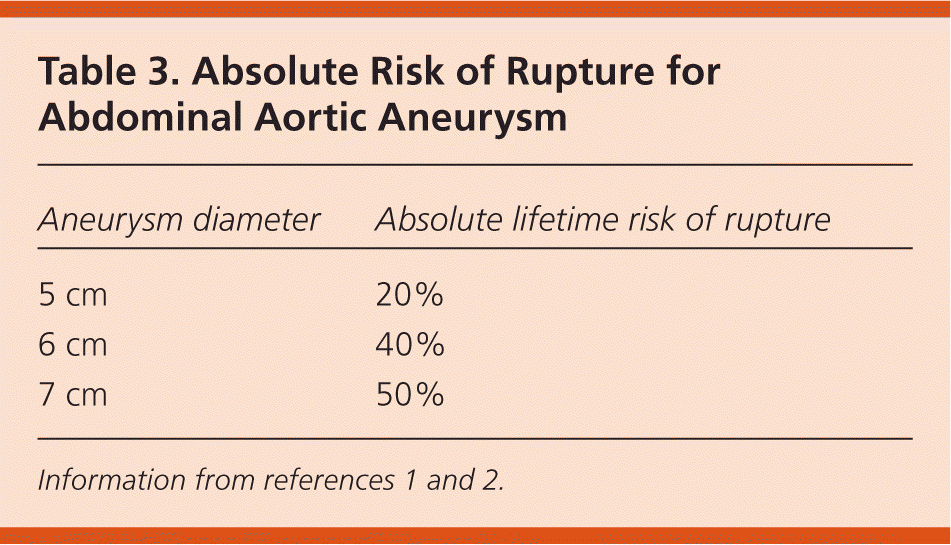
| Aneurysm diameter | Absolute lifetime risk of rupture |
|---|---|
| 5 cm | 20% |
| 6 cm | 40% |
| 7 cm | 50% |
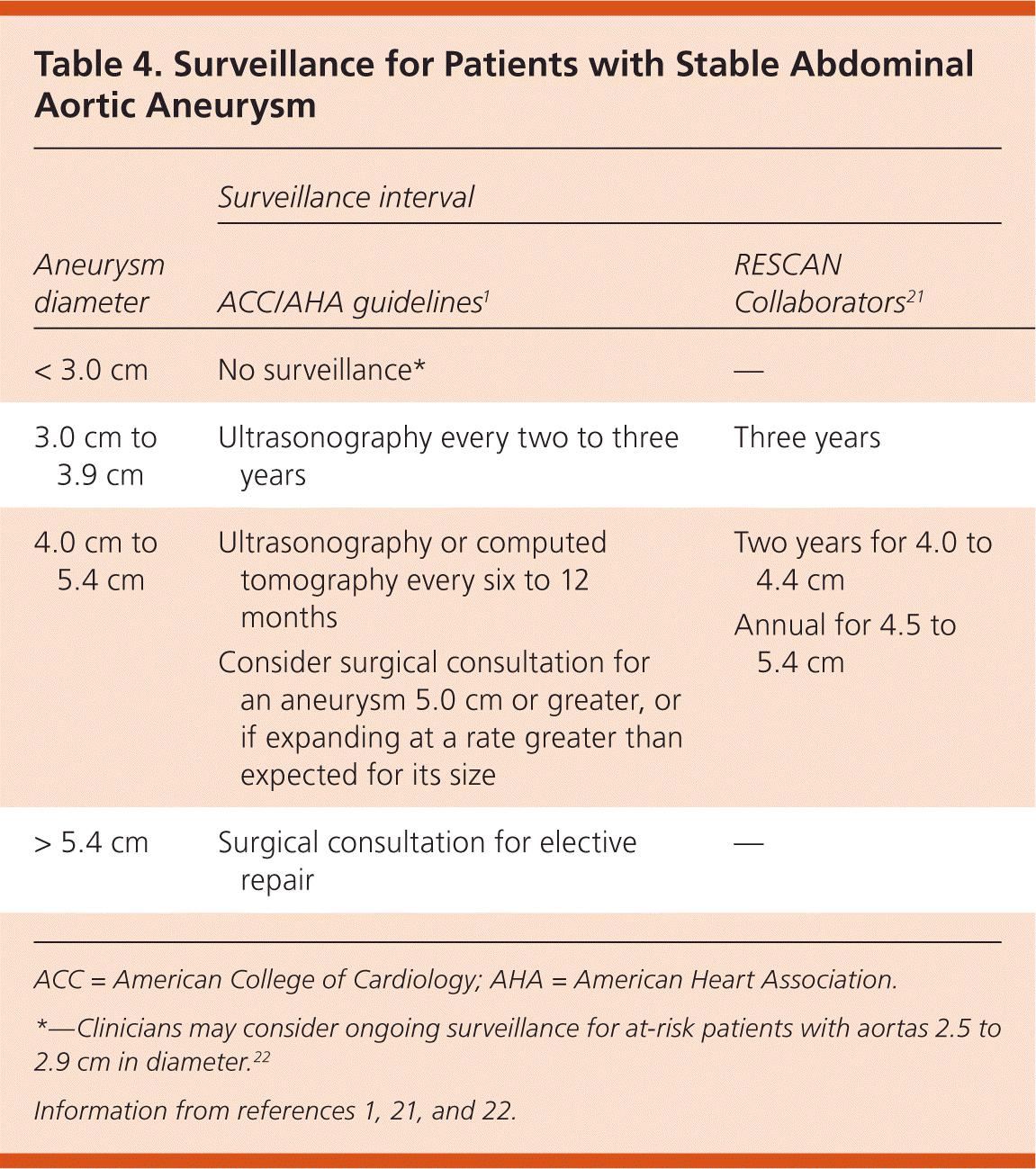
| Surveillance interval | ||
|---|---|---|
| Aneurysm diameter | ACC/AHA guidelines1 | RESCAN Collaborators21 |
| < 3.0 cm | No surveillance* | — |
| 3.0 cm to 3.9 cm | Ultrasonography every two to three years | Three years |
| 4.0 cm to 5.4 cm | Ultrasonography or computed tomography every six to 12 months | Two years for 4.0 to 4.4 cm |
| Consider surgical consultation for an aneurysm 5.0 cm or greater, or if expanding at a rate greater than expected for its size | ||
| > 5.4 cm | Surgical consultation for elective repair | — |
Several studies have compared the outcomes of early elective surgery vs. ongoing radiographic monitoring (by computed tomography or ultrasonography) for aneurysms between 3.0 and 5.5 cm. Surgery in the surveillance groups occurred only when the aneurysm exceeded 5.5 cm, expanded by more than 1 cm per year (another risk factor for rupture), or became tender or symptomatic.23 At later points in the follow-up period, there was some weak evidence that suggested a benefit to early surgical repair. The authors noted that this is possibly because those who underwent early surgery were more motivated to make lifestyle changes that may improve AAA-related outcomes, including a higher rate of smoking cessation.23 Overall, these studies suggest that the risks of operative management do not exceed mortality benefits, and that survival is not improved by elective surgery for aneurysms smaller than 5.5 cm.24
Current guidelines do not advocate rescreening persons with an aortic diameter smaller than 3.0 cm.9,11 In the Multi-centre Aneurysm Screening Study, persons with negative screening results (smaller than 3.0 cm diameter) were not rescreened later. The rate of AAA rupture in this group did increase over the duration of the study; however, the increase was not enough to offset the continued reduction in AAA-mortality in the study overall.11 Conversely, a prospective cohort study of men 67 to 74 years of age found that those with aortic diameters of 2.5 to 2.9 cm, also known as aortic ectasia, on initial screening had an increased risk of subsequent AAA diagnosis compared with those who had aortas measuring 2.4 cm or less. Thus, these persons could be considered for retesting, although present data do not support the cost effectiveness of this approach.11,22
Treatment
MEDICAL
Several nonsurgical options have been studied for the potential ability to slow aneurysm progression. Smoking cessation may help because smoking causes an incremental increased growth rate of up to 0.4 mm per year.25 In terms of pharmacologic therapy, statins, antihypertensives, and antibiotics have been studied. Beta blockers are known to improve perioperative mortality for AAA repair; however, randomized trial results indicate that their effects on AAA enlargement are not significant.
Other antihypertensives (e.g., angiotensin-converting enzyme inhibitors) also do not appear to be effective.26,27 Although there have been recommendations supporting statin use, the evidence for reducing AAA growth or rupture has been poor, and better-quality studies do not indicate a direct benefit.27,28 Statins are likely to be used for overall cardiovascular risk reduction and do improve all-cause mortality in patients after AAA repair.28 Additionally, roxithromycin (a macrolide antibiotic not available in the United States) and doxycycline have weak evidence for inhibiting AAA growth, because secondary infection in the aortic wall, likely from Chlamydophila pneumoniae, may promote AAA progression.27,29
SURGICAL
Elective Repair of Stable AAA. A diameter of 5.5 cm has been used in many protocols as a threshold for performing elective surgery, particularly for infrarenal and juxtarenal aneurysms. At this size, it is thought that the benefits of surgery outweigh the risks. Open and endovascular repair are the two main approaches. Multiple studies have shown that there is no significant difference between the two approaches in terms of overall long-term mortality.30,31 Open repair carries a 30-day mortality risk between 4% and 5%. The less-invasive endovascular approach has gained favor because of improved early outcomes, with a 30-day mortality risk between 1% and 2%.30 However, studies have shown that the mortality benefits initially reported with endovascular repair are essentially gone by two to three years postprocedure.30–32
In addition, patients undergoing endovascular repair have a higher rate of graft complications and need for secondary interventions compared with patients undergoing open repair. This may make endovascular repair less cost-effective in the long term.30 The patient's age may also play a role in which procedure is more beneficial. One study demonstrated improved survival with endovascular repair in patients younger than 70 years, whereas patients 70 years or older tended to do better with open repair.32
Emergent Repair of Ruptured AAA. Ruptured AAAs cause an estimated 4% to 5% of sudden deaths in the United States. Up to 50% of patients with ruptured AAAs do not reach the hospital, and those who do survive to the operating room have a mortality rate as high as 50%.33 Much like elective repair, studies thus far have not identified a statistically significant difference in survival with endovascular vs. open repair of ruptured AAA.34 Factors that appear to impact survival include decreased time from presentation to operative intervention, and the presence of a surgical team experienced in AAA repair.35
Data Sources: A PubMed search was completed in Clinical Queries using the key terms abdominal aortic aneurysm, diagnosis, evaluation, and treatment. Also searched were Essential Evidence Plus and the Cochrane database. The search included meta-analyses, randomized controlled trials, and reviews. Search dates: January 13, 2012; May 13, 2012; and August 18, 2014.