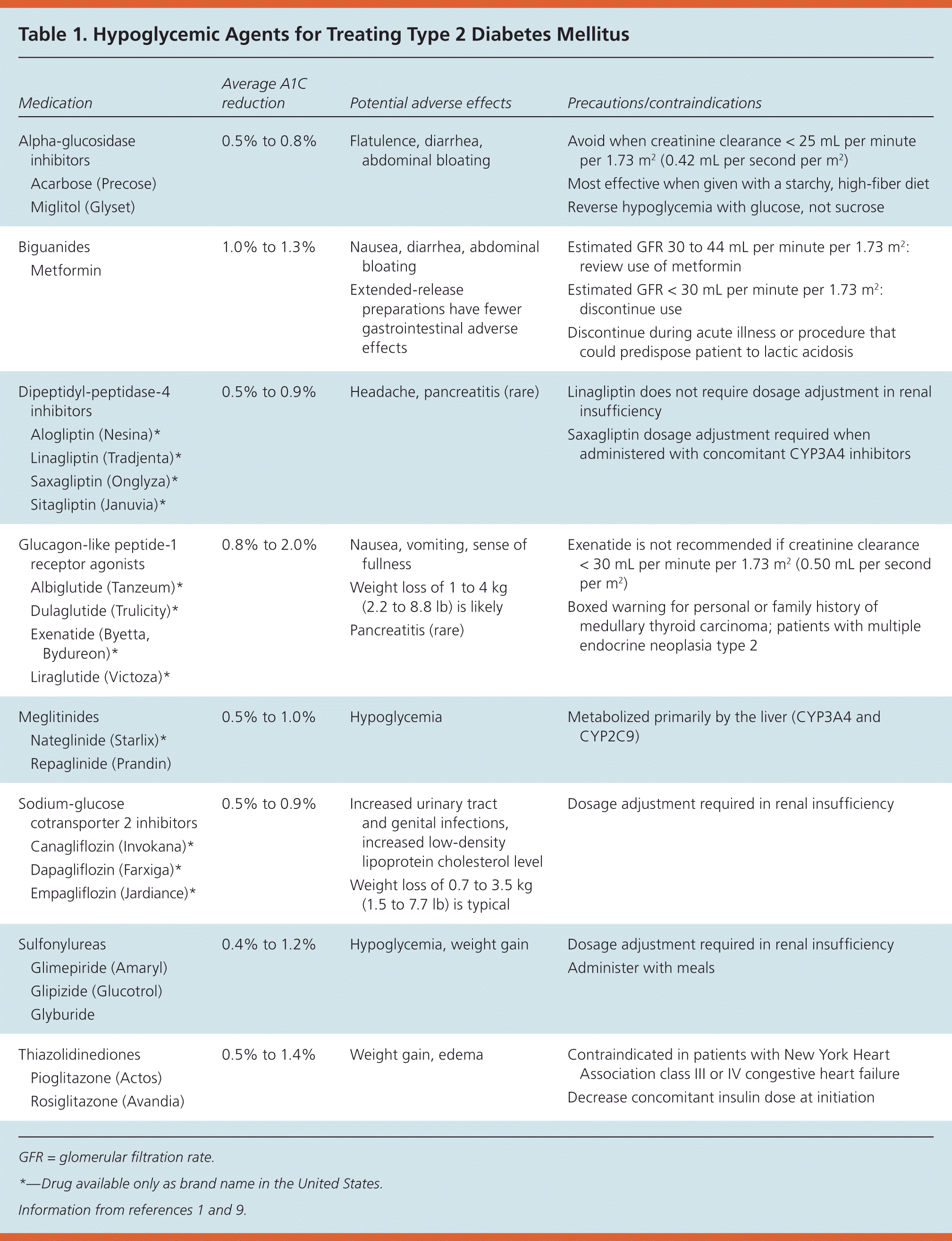
Acarbose (Precose) Miglitol (Glyset)
| | | Avoid when creatinine clearance < 25 mL per minute per 1.73 m2 (0.42 mL per second per m2) Most effective when given with a starchy, high-fiber diet Reverse hypoglycemia with glucose, not sucrose
|
| | Nausea, diarrhea, abdominal bloating Extended-release preparations have fewer gastrointestinal adverse effects
| Estimated GFR 30 to 44 mL per minute per 1.73 m2: review use of metformin Estimated GFR < 30 mL per minute per 1.73 m2: discontinue use Discontinue during acute illness or procedure that could predispose patient to lactic acidosis
|
Alogliptin (Nesina)* Linagliptin (Tradjenta)* Saxagliptin (Onglyza)* Sitagliptin (Januvia)*
| | | |
| | Nausea, vomiting, sense of fullness Weight loss of 1 to 4 kg (2.2 to 8.8 lb) is likely Pancreatitis (rare)
| Exenatide is not recommended if creatinine clearance < 30 mL per minute per 1.73 m2 (0.50 mL per second per m2) Boxed warning for personal or family history of medullary thyroid carcinoma; patients with multiple endocrine neoplasia type 2
|
Nateglinide (Starlix)* Repaglinide (Prandin)
| | | |
| | Increased urinary tract and genital infections, increased low-density lipoprotein cholesterol level Weight loss of 0.7 to 3.5 kg (1.5 to 7.7 lb) is typical
| |
Glimepiride (Amaryl) Glipizide (Glucotrol) Glyburide
| | | |
Pioglitazone (Actos) Rosiglitazone (Avandia)
| | | |