
Am Fam Physician. 2015;92(4):304-305
Author disclosure: No relevant financial affiliations.
Vorapaxar (Zontivity) is an antiplatelet drug labeled for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or who have peripheral arterial disease. Unlike other platelet inhibitors, it blocks the protease-activated receptor-1, which is expressed on platelets and reacts to thrombin to cause platelet aggregation.1
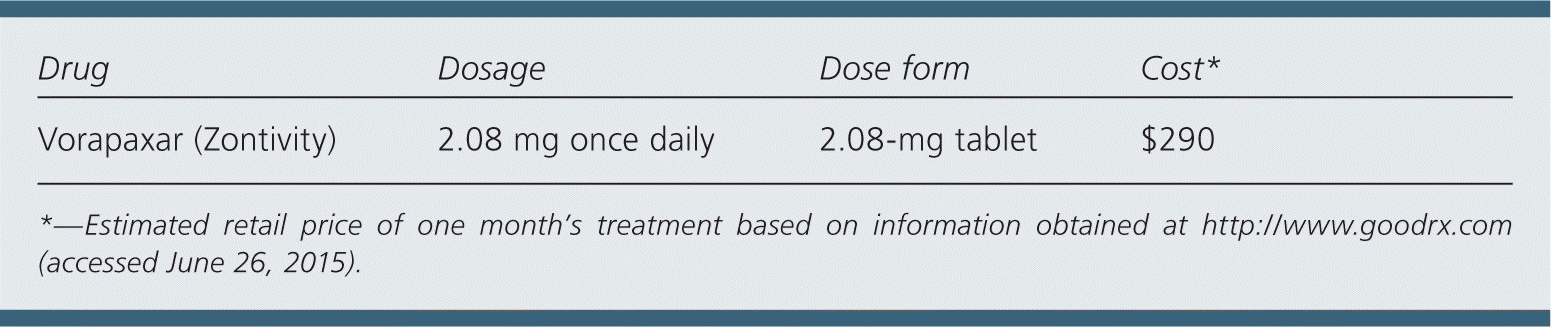
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Vorapaxar (Zontivity) | 2.08 mg once daily | 2.08-mg tablet | $290 |
SAFETY
Bleeding is the most serious safety concern. Vorapaxar is contraindicated in patients with a history of stroke, transient ischemic attack, or intracranial hemorrhage, and in those with active bleeding caused by a peptic ulcer. Taking vorapaxar increases the risk of intracranial hemorrhage, clinically significant bleeding (i.e., bleeding requiring a blood transfusion, bleeding causing hemodynamic compromise, bleeding that prolongs hospitalization, or intracerebral bleeding), and fatal bleeding. One additional clinically significant bleeding event occurs for every 16 patients treated (number needed to harm = 16 over three years). Vorapaxar is metabolized slowly, so it cannot be discontinued immediately before procedures to reduce bleeding. It is a U.S. Food and Drug Administration pregnancy category B drug.
TOLERABILITY
Vorapaxar is well tolerated by most patients. Rare adverse effects include diplopia and retinopathy. In clinical trials, dropout rates were equal to those with placebo (about 25%).
EFFECTIVENESS
Two large randomized controlled trials compared aspirin and/or clopidogrel (Plavix) with or without vorapaxar in more than 39,000 patients.2,3 When patients with MI, stroke, or peripheral arterial disease add vorapaxar to standard antiplatelet therapy for three years, the risk of the composite outcome of death from cardiovascular causes, MI, stroke, or need for urgent coronary catheterization decreases from 11.8% to 10.1%. In patients with a history of MI or peripheral arterial disease, only the rate of MI is significantly decreased, from 6.1% to 5.2% (number needed to treat = 111 for three years). Vorapaxar does not significantly decrease the individual rates of urgent coronary catheterization, stroke, cardiovascular death, or death from any cause in these patients.2 In a study of patients with acute coronary syndrome without ST elevation, vorapaxar increased rates of recurrent ischemia, urgent revascularization, and death from any cause.3 It should not be used in these patients.
PRICE
A one-month supply of vorapaxar costs $290. This is in addition to the cost of aspirin and clopidogrel therapy.
SIMPLICITY
Vorapaxar is taken once daily with or without food. It should be used in combination with standard dosages of aspirin and clopidogrel. Vorapaxar is metabolized by the liver and should not be taken by patients who are also taking medicines that induce or inhibit the cytochrome P450 3A hepatic enzyme, such as carbamazepine (Tegretol) or ketoconazole.1
Bottom Line
Vorapaxar should be limited to select patients with a history of MI or peripheral arterial disease who desire additional treatment for the prevention of MI. Added to aspirin and clopidogrel, vorapaxar provides a small additional benefit but also significantly increases the risk of serious bleeding.2 It should not be used in patients with acute coronary syndrome without ST elevation or in patients with a history of stroke, transient ischemic attack, or intracranial hemorrhage.1,3
