
Am Fam Physician. 2016;93(9):764-771
Patient information: See related handout on prostate cancer treatment, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
This summary of the American Cancer Society Prostate Cancer Survivorship Care Guidelines targets primary care physicians who coordinate care of prostate cancer survivors with subspecialists. Prostate cancer survivors should undergo prostate-specific antigen screening every six to 12 months and digital rectal examination annually. Surveillance of patients who choose watchful waiting for their prostate cancer should be conducted by a subspecialist. Any hematuria or rectal bleeding must be thoroughly evaluated. Prostate cancer survivors should be screened regularly for urinary incontinence and sexual dysfunction. Patients with predominant urge incontinence symptoms, which can occur after surgical and radiation treatments, may benefit from an anticholinergic agent. If there is difficulty with bladder emptying, a trial of an alpha blocker may be considered. A phosphodiesterase type 5 inhibitor can effectively treat sexual dysfunction following treatment for prostate cancer. Osteoporosis screening should occur before initiation of androgen deprivation therapy, and patients treated with androgen deprivation therapy should be monitored for anemia, metabolic syndrome, and vasomotor symptoms. Healthy lifestyle choices should be encouraged, including weight management, regular physical activity, proper nutrition, and smoking cessation. Primary care physicians should be vigilant for psychosocial distress, including depression, among prostate cancer survivors, as well as the potential impact of this distress on patients' family members and partners.
Prostate cancer is commonly recognized and treated. In 2014, there were an estimated 3 million prostate cancer survivors in the United States.1 The number of treated prostate cancer survivors is growing, and this population is aging. These survivors are routinely part of a primary care physician's panel; however, nearly one-half of primary care physicians feel unprepared to manage the long-term effects of prostate cancer treatment.2 In this summary, we review the July 2014 American Cancer Society (ACS) Prostate Cancer Survivorship Care Guidelines, which are based on recommendations from an interdisciplinary expert workgroup.3 Although other guidelines have addressed aspects of follow-up for prostate cancer treatment, this is the first time comprehensive prostate cancer survivorship guidelines have been published. The ACS guidelines were developed to assist primary care physicians in caring for prostate cancer survivors, including monitoring for cancer recurrence, and managing the physical and psychological consequences of treatment.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Digital rectal examination should be performed annually as follow-up after prostate cancer treatment. | C | 3, 5 |
| Prostate-specific antigen levels should be checked every six to 12 months for five years and then annually thereafter as follow-up after prostate cancer treatment. | C | 3, 5 |
| In patients treated with androgen deprivation therapy, baseline dual energy x-ray absorptiometry should be performed to measure bone mineral density. | C | 3, 13, 14 |
| After prostate cancer treatment, health-related quality of life should be assessed annually using tools such as the Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC-CP; eFigure A). | C | 3, 20 |
Epidemiology
Prostate cancer is relatively common, and survival rates are exceedingly high. In 2014, it was estimated that 233,000 new cases would be diagnosed within that year.1 Up to 93% of prostate cancers are diagnosed in a local or regional stage,1 which confers a nearly 100% five-year survival rate.3 As a result, prostate cancer survivors are treated in primary care clinics for years and even decades after treatment.
Coordination of Care
To facilitate appropriate coordination of care for prostate cancer survivors, the Institute of Medicine recommends that subspecialists provide primary care physicians with treatment summaries and a follow-up plan.4 Primary care physicians should collaborate with treating subspecialists to define their respective roles and responsibilities, taking into account patients' specific situations, including social support and geographic limitations. Currently, there is no systematic approach to information-sharing between subspecialists involved in cancer care and primary care physicians.1 Each physician may need to develop his or her own system to ensure appropriate coordination.
Monitoring for Prostate Cancer Recurrence
Patients commonly choose active surveillance of their prostate cancer. Surveillance for cancer recurrence is typically performed by a subspecialist 3 and should be clearly handed off to the primary care physician when the subspecialist will no longer follow the patient. It is important to note that following prostate cancer survivors for cancer recurrence is distinct from screening for new cancers.
The guidelines recommend lifelong monitoring for recurrence using blood prostate-specific antigen (PSA) levels and digital rectal examination (DRE). Either the subspecialist or the primary care physician should perform a DRE annually. The PSA level should be checked every six to 12 months for the first five years and then annually thereafter. After radical prostatectomy, any measurable level of PSA (0.03 ng per mL [0.03 mcg per L] is considered undetectable) warrants referral to the subspecialist. After radiation therapy, the PSA level reaches its lowest point six months to several years after treatment. The goal for patients treated with radiation is a PSA level less than 1.0 ng per mL (1.0 mcg per L). Any subsequent rise in PSA level warrants referral to the subspecialist (Table 1).3,5
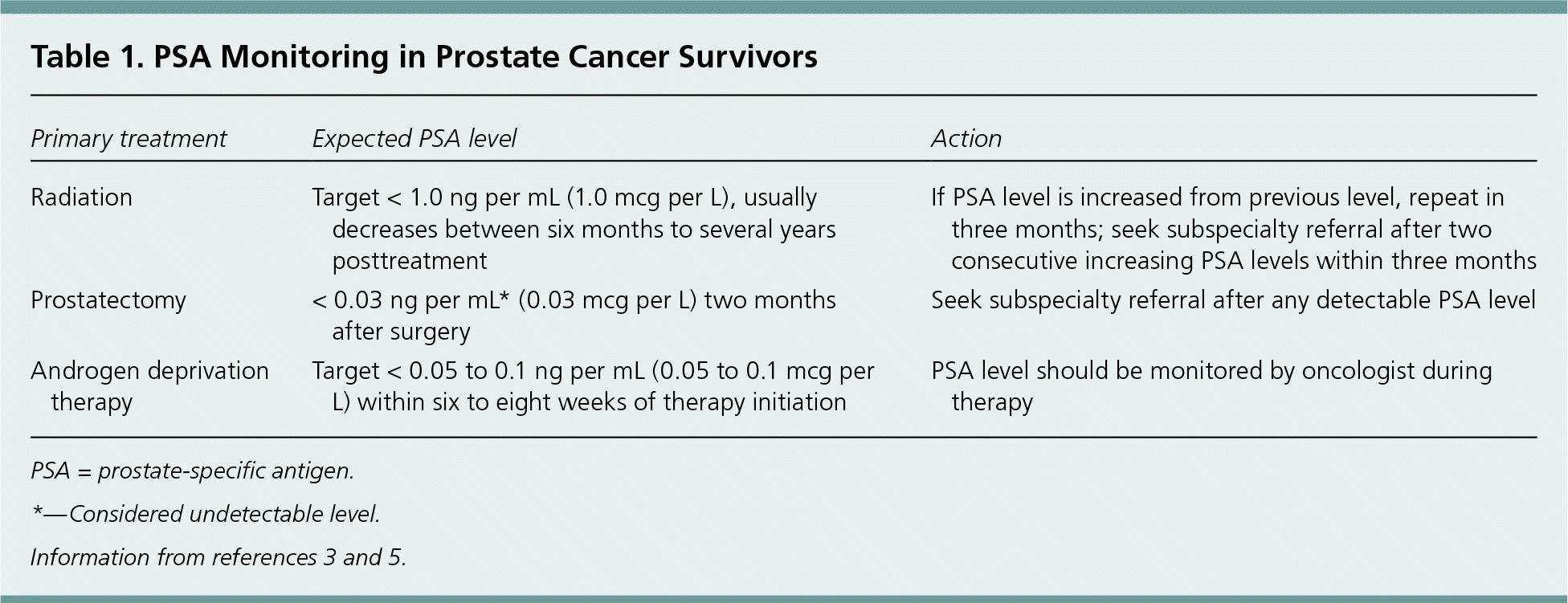
| Primary treatment | Expected PSA level | Action |
|---|---|---|
| Radiation | Target < 1.0 ng per mL (1.0 mcg per L), usually decreases between six months to several years posttreatment | If PSA level is increased from previous level, repeat in three months; seek subspecialty referral after two consecutive increasing PSA levels within three months |
| Prostatectomy | < 0.03 ng per mL* (0.03 mcg per L) two months after surgery | Seek subspecialty referral after any detectable PSA level |
| Androgen deprivation therapy | Target < 0.05 to 0.1 ng per mL (0.05 to 0.1 mcg per L) within six to eight weeks of therapy initiation | PSA level should be monitored by oncologist during therapy |
Monitoring for Secondary Malignancies
Secondary malignancies after radiation therapy occur relatively infrequently, at a rate of one in 220 to 290,6 with bladder and rectal cancers most commonly observed.7 Any episode of hematuria presenting in a prostate cancer survivor should prompt referral for cystoscopy to exclude bladder cancer. Routine urinalysis to screen for bladder cancer is not recommended. Any episode of rectal bleeding should trigger a workup to exclude rectal or colon cancer.3
Assessment of Physical and Psychosocial Issues
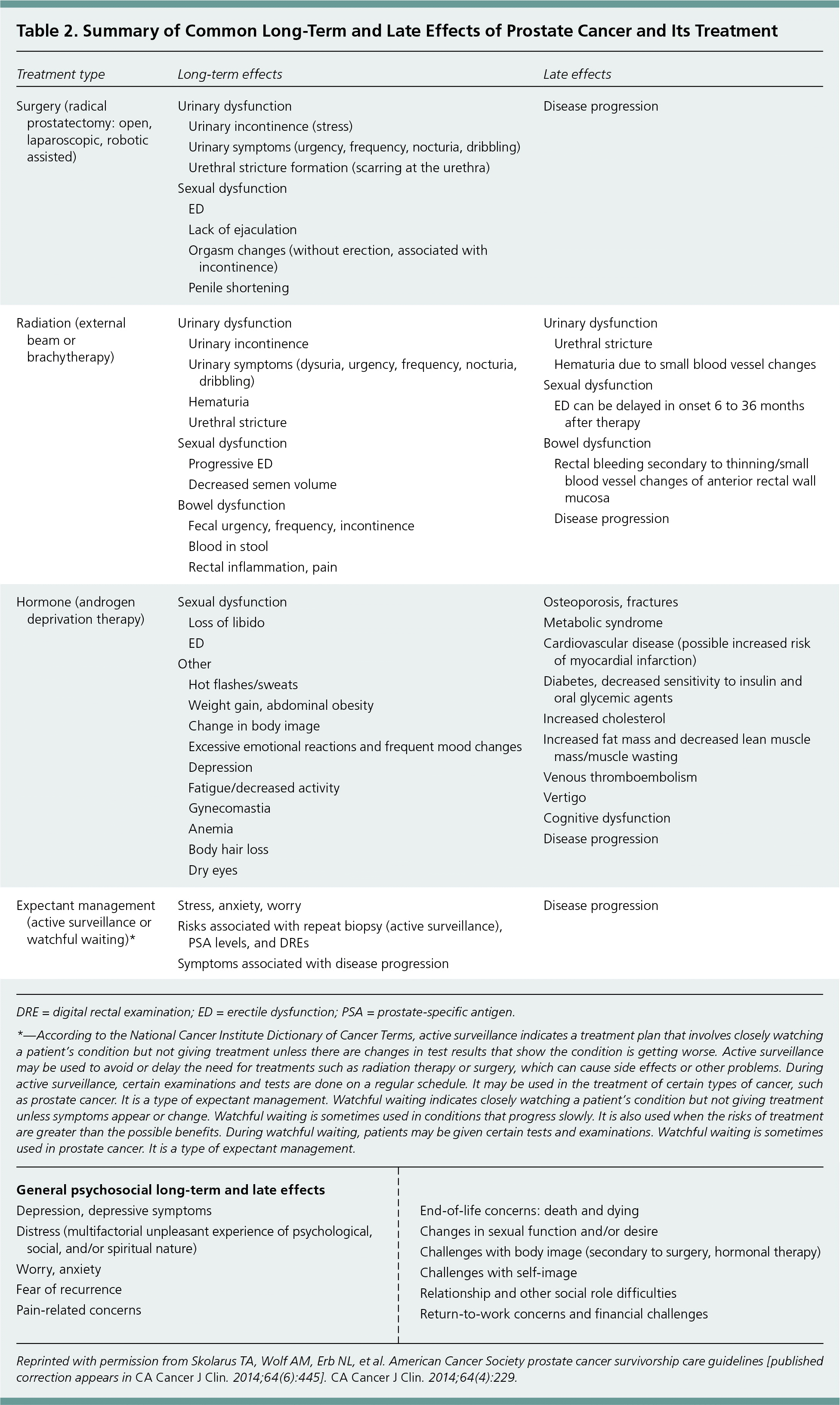
| Treatment type | Long-term effects | Late effects | ||
|---|---|---|---|---|
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
| General psychosocial long-term and late effects | ||||
| Depression, depressive symptoms | ||||
| Distress (multifactorial unpleasant experience of psychological, social, and/or spiritual nature) | ||||
| Worry, anxiety | ||||
| Fear of recurrence | ||||
| Pain-related concerns | ||||
| End-of-life concerns: death and dying | ||||
| Changes in sexual function and/or desire | ||||
| Challenges with body image (secondary to surgery, hormonal therapy) | ||||
| Challenges with self-image | ||||
| Relationship and other social role difficulties | ||||
| Return-to-work concerns and financial challenges | ||||

| Anemia: specific risk for men receiving ADT |
| Perform annual CBC to monitor hemoglobin levels |
| Bowel dysfunction |
| Discuss bowel function and symptoms (e.g., rectal bleeding) with survivors |
| For men with a negative colorectal cancer screening result, prescribe stool softeners, topical steroids, or anti-inflammatories for survivors experiencing rectal bleeding |
| Refer survivors with persistent rectal symptoms (e.g., bleeding, sphincter dysfunction, rectal urgency and frequency) to the appropriate subspecialist |
| Cardiovascular and metabolic effects: specific risk for men receiving ADT |
| Follow USPSTF guidelines for evaluation and screening for cardiovascular risk factors, blood pressure monitoring, lipid profiles, and serum glucose (http://www.uspreventiveservicestaskforce.org/uspstopics.htm) |
| Distress/depression/PSA anxiety |
| Assess for distress/depression/PSA anxiety periodically (at least annually) using a simple screening tool, such as the Distress Thermometer |
| Manage distress/depression using in-office counseling resources or pharmacotherapy as appropriate |
| If office-based counseling and treatment are insufficient, refer survivors experiencing distress/depression for further evaluation and/or treatment by appropriate subspecialists |
| Fracture risk/osteoporosis: specific risk for men receiving ADT |
| Assess risk of fracture for men treated with ADT or older radiation techniques through baseline DEXA scan and calculation of a FRAX score |
| For men determined to be high risk, prescribe weekly bisphosphonate therapy (oral alendronate at a dosage of 70 mg) or annual intravenous zoledronic acid at a dosage of 5 mg to increase bone density; denosumab is also approved by the FDA to treat men at increased risk of osteoporosis |
| Sexual dysfunction/body image |
| Discuss sexual function with survivors |
| Use validated tools, such as the SHIM, to monitor erectile function over time |
| Erectile dysfunction may be addressed through a variety of options, including penile rehabilitation or prescription of phosphodiesterase type 5 inhibitors (e.g., sildenafil, vardenafil, tadalafil) |
| Refer men with persistent sexual dysfunction to a urologist, sexual health specialist, or psychotherapist to review treatment and counseling options |
| Sexual intimacy |
| Encourage couples to discuss their sexual intimacy and refer to counseling or support services as appropriate |
| Prescribe medication as described above to address erectile dysfunction |
| Instruct couples on use of sexual aids to improve erectile dysfunction for men/male partners as well as postmenopausal symptoms for women; refer to health professional with expertise in sex therapy |
| Urinary dysfunction |
| Discuss urinary function (e.g., urinary stream, difficulty emptying bladder) and incontinence with all survivors |
| Consider timed voiding, prescribing anticholinergic medications (e.g., oxybutynin) to address issues such as nocturia, frequency, or urgency; consider alpha blockers (e.g., tamsulosin) for slow stream |
| Refer survivors with postprostatectomy incontinence to a physical therapist for pelvic floor rehabilitation; at a minimum, instruct survivors about Kegel exercises |
| Refer men with persistent leakage or other urinary symptoms to a urologist for further evaluation (e.g., urodynamic testing, cystoscopy) and discussion of treatment options, including surgical placement of a male urethral sling or artificial urinary sphincter for incontinence |
| Vasomotor symptoms (e.g., hot flashes): specific risk for men receiving ADT |
| Although not approved by the FDA for this indication, prescription of selective serotonin or noradrenergic reuptake inhibitors or gabapentin may offer symptom relief |
PHYSICAL ISSUES
Urinary Incontinence. Stress incontinence typically improves during the first year after prostatectomy and then usually resolves.8 Radiation therapy may cause short-term effects that are indistinguishable from symptoms of urinary tract infection. Long-term effects of radiation therapy are generally more complex, and may include lower urinary tract symptoms similar to those of benign prostatic hyperplasia, as well as anatomic changes such as strictures, fistulas of the urinary tract, overt urinary incontinence, or hematuria.3 Although hematuria may be a long-term adverse effect from radiation treatment, it warrants subspecialty referral for cystoscopy. It is important for physicians to inquire about symptoms of urinary leakage, frequency, or incontinence, and the use of incontinence pads because patients are often hesitant to discuss these symptoms.
Patients with predominant urge incontinence symptoms, which can occur following surgical and radiation treatments, may benefit from an anticholinergic agent (e.g., oxybutynin). If there is difficulty with bladder emptying, a trial of an alpha blocker (e.g., tamsulosin [Flomax]) may be considered. Multiple approaches exist to address postprostatectomy incontinence. These include less invasive methods, such as pelvic floor muscle exercises and medication management, or more invasive procedures using urethral bulking agents, such as collagen, slings, or artificial urinary sphincters.8
Sexual Dysfunction. Sexual dysfunction after prostate cancer treatment with radiation and prostatectomy is common. Rates of postprostatectomy erectile dysfunction (ED) have been reported up to 79% at two years and 76% at five years, whereas rates of postradiation ED have been reported up to 61% at two years and 72% at five years.9 Prostate cancer survivors at higher risk of sexual dysfunction include older men, those who already have ED, and those who did not have nerve-sparing surgery.10
A phosphodiesterase type 5 (PDE5) inhibitor can treat sexual dysfunction after prostate cancer treatment.11 The evidence supporting early penile rehabilitation to improve function, including administration of a PDE5 inhibitor soon after surgery, is equivocal.12 If ED does not improve with a PDE5 inhibitor, additional trials should be considered because many men can regain some erectile function up to two to four years after surgery.3 ED can develop six to 36 months after radiation therapy is complete. A trial of a PDE5 inhibitor is warranted for patients with ED after radiation therapy.3
If PDE5 inhibitors do not successfully treat ED, other treatment options may be considered in consultation with a urologist. These include pharmacologic interventions, such as intraurethral dissolvable prostaglandin pellets and intracavernosal prostaglandin injections, or mechanical interventions, such as vacuum erection devices and penile prostheses.3
Osteoporosis. Androgen deprivation therapy increases the risk of decline in bone mineral density and fracture.13 Patients should be counseled on appropriate calcium intake of up to 1,200 mg per day and vitamin D intake of at least 600 IU per day, both of which are consistent with the guidelines' recommendations for nutritional support. Bone density evaluation is commonly overlooked in prostate cancer survivors. Only about 10% of men receiving androgen deprivation therapy have a bone density evaluation six months before or 12 months after starting treatment.14 If androgen deprivation therapy is planned, a baseline measurement of bone mineral density using dual energy x-ray absorptiometry followed by a Fracture Risk Assessment Tool (FRAX) score (https://www.shef.ac.uk/FRAX/tool.jsp) should be obtained before initiating treatment. The FRAX score should take into account previous use of androgen deprivation therapy. Bisphosphonate therapy should be initiated if indicated based on the FRAX score.3
Bowel Dysfunction. Bowel dysfunction is a concern after treatment with radiation therapy. Radiation can cause acute or chronic proctitis with bowel symptoms including irregular bowel movements, flatulence, diarrhea, and rectal bleeding. Hydration, stool softeners, topical steroids (e.g., hydrocortisone), or anti-inflammatories (e.g., mesalamine) may provide relief. Late effects may include rectal ulceration, fistula formation, sphincter dysfunction, rectal urgency, frequency, and rectal pain. These late effects may require referral to a subspecialist.3,15
Cardiovascular and Metabolic Effects. The data are inconclusive to determine the risk of cardiovascular disease and metabolic effects after androgen deprivation therapy. Treatment with androgen deprivation therapy may increase the risk of metabolic syndrome by decreasing lean muscle mass, increasing fat mass, decreasing insulin sensitivity, and adversely impacting cholesterol markers.17 No specific interventions are recommended, but routine monitoring of cardiovascular risk factors, including blood pressure, glucose levels, and cardiovascular risk factor screening, should be considered according to guidelines for the general population. This is particularly important in patients treated with androgen deprivation therapy for six months or more because they have increased propensity to develop metabolic syndrome.3
Vasomotor Symptoms. The prevalence of hot flashes may reach 40% in men treated with androgen deprivation therapy.18 Vasomotor symptoms can last for years after therapy is completed. Although there is no treatment approved by the U.S. Food and Drug Administration for vasomotor symptoms, the ACS guidelines recommend off-label use of paroxetine (Paxil), 10 mg per day, or venlafaxine, 37.5 mg per day. Gabapentin (Neurontin) may also be considered as a treatment option.3
PSYCHOSOCIAL ISSUES
Prostate cancer survivors should be assessed for psychosocial issues related to their cancer and cancer treatments.3 This assessment should take into account the specific therapy received. Health-related quality of life should be tracked annually to measure the survivor's perceived physical and psychosocial health. The Centers for Disease Control and Prevention defines health-related quality of life as a broad multidimensional concept that usually includes self-reported measures of physical and mental health.19 There is evidence to suggest a decrease in health-related quality of life after diagnosis of prostate cancer, particularly in the first six months.20 Although health-related quality of life generally improves over time, many survivors are at risk of developing major depressive disorder.20 There are several tools available to help assess these issues, including the Sexual Health Inventory for Men and the Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC-CP; eFigure A), which provides the broadest physical and psychosocial assessment. These tools are available within the original guidelines, which are accessible for free at http://onlinelibrary.wiley.com/doi/10.3322/caac.21234/abstract.
Depression, Anxiety, and Psychological Distress. Between 9% and 25% of prostate cancer survivors experience major depressive disorder, and at least 25% have increased anxiety.21,22 Many experience psychological distress, including worsening social and emotional functioning.23 These symptoms can contribute to increased health care use and costs,22 and increased rates of suicidal ideation and suicide completion.24 Routine screening for psychological distress is advised, including discussions with family and caregivers. Risk factors for psychological distress include living alone, younger age, lower educational attainment, advanced prostate disease, multiple comorbidities, history of psychiatric diagnoses, and low cognitive or physical function.3 Anxiety related to PSA surveillance has also been reported.25 Referral for behavioral interventions may be warranted among persons experiencing significant psychosocial distress.
Relationships. Partners of prostate cancer survivors should be considered in survivorship care. Partners experience stress related to diagnosis and treatment, which can adversely affect the relationship.26 The ACS recommends that partners be included in shared decision making for interventions to manage ED, anxiety and depression, and adverse effects of androgen deprivation therapy. Referral for counseling or sex therapy may also be beneficial.
Healthy Lifestyle Choices
HEALTHY WEIGHT AND ACTIVITY
Although physical activity is important for the general population, prostate cancer survivors should pay special attention to their activity level. Evidence suggests a decreased risk of cancer recurrence among those who are physically active.27 The ACS recommendations for physical activity are similar to those of the American Heart Association and include at least 150 minutes per week of moderate-intensity or 75 minutes per week of vigorous-intensity aerobic exercise, which may also include weight-bearing activities.3 Cancer survivors are encouraged to avoid any period of inactivity and return to normal activities as soon as possible after surgery or other interventions.
NUTRITION
The ACS recommends a diet plentiful in micronutrient- and phytochemical-rich fruits and vegetables for prostate cancer survivors. This diet should provide 600 IU per day of vitamin D and up to 1,200 mg of calcium. Alcohol consumption should be limited to two drinks per day. Patients with absorption problems should be referred to a registered dietitian, preferably with additional expertise as a certified specialist in oncology nutrition.
SMOKING
Smoking is associated with an increased risk of recurrence of prostate cancer with metastatic disease in men who have undergone prostatectomy.28 Smoking cessation should be discussed with all prostate cancer survivors, similar to the general population.
Data Sources: In preparing this summary of the ACS guidelines, we also reviewed literature that supports the ACS guideline. Many of the supporting references were previously cited in the guideline. A PubMed search was also completed. Key search terms included prostate, prostatectomy, radiation, androgen deprivation therapy, ADT, incontinence, osteoporosis, survivorship, survivor, anemia, PSA, prostate specific antigen, digital rectal exam, and DRE. Additionally, we used an Essential Evidence Plus search, as well as UpToDate and the Cochrane Database of Systematic Reviews. Search dates: November 17, 2014, through March 5, 2015, and February 9, 2016.