
Am Fam Physician. 2016;94(1):53-58
Author disclosure: No relevant financial affiliations.
Clinical Question
Is the use of a procalcitonin-guided antibiotic therapy algorithm safe and effective for reducing antibiotic use in patients with acute respiratory infections?
Evidence-Based Answer
A procalcitonin-guided antibiotic therapy algorithm should be used to decrease antibiotic use in adults with acute respiratory infections. (Strength of Recommendation [SOR]: A, based on a meta-analysis of multiple randomized controlled trials [RCTs].) The use of a procalcitonin-guided therapy algorithm reduces antibiotic use by 3.47 days without increasing morbidity or mortality in adults with acute respiratory infections. In the primary care setting, the use of procalcitonin-guided therapy algorithms decreases the rate of antibiotic prescription by 72% without affecting the risk of treatment failure. In children with lower respiratory tract infections, procalcitonin guidance should be used to reduce the duration of antibiotic therapy. (SOR: B, based on a single RCT.)
Evidence Summary
A Cochrane review and meta-analysis of 14 RCTs in primary care, emergency department, and intensive care unit settings included a total of 4,221 patients.1 In each trial, researchers randomized adults presenting with acute respiratory infections to procalcitonin-guided antibiotic therapy or standard care. All studies used a procalcitonin algorithm to guide antibiotic initiation (Table 1), and some also used the algorithm to guide discontinuation. Patients in the procalcitonin group received 3.47 fewer days of antibiotic treatment (95% confidence interval [CI], −3.78 to −3.17), with no difference in 30-day mortality (odds ratio [OR] = 0.94; 95% CI, 0.71 to 1.23) or treatment failure (OR = 0.82; 95% CI, 0.67 to 1.01).
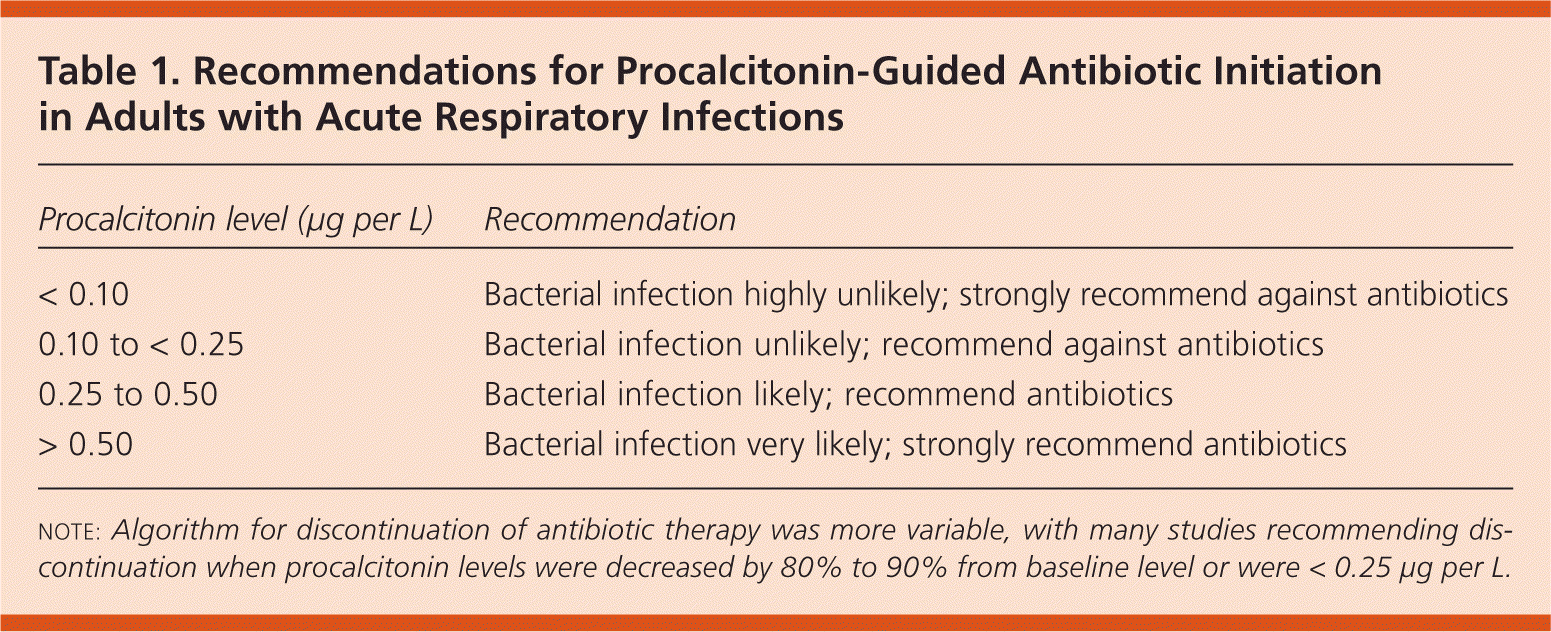
| Procalcitonin level (μg per L) | Recommendation |
|---|---|
| < 0.10 | Bacterial infection highly unlikely; strongly recommend against antibiotics |
| 0.10 to < 0.25 | Bacterial infection unlikely; recommend against antibiotics |
| 0.25 to 0.50 | Bacterial infection likely; recommend antibiotics |
| > 0.50 | Bacterial infection very likely; strongly recommend antibiotics |
The authors of the Cochrane review performed multiple sensitivity analyses that excluded trials from the intensive care unit, trials with the potential for significant bias, and trials with poor adherence to the algorithm. The analyses yielded similar results, suggesting that the findings are robust. Results were similar when stratified by treatment setting and by type of respiratory infection (upper respiratory infections including the common cold, rhinosinusitis, otitis, tonsillitis, and pharyngitis, and lower respiratory tract infections including community-, hospital-, and ventilator-acquired pneumonia and bronchitis). A major limitation was relatively short follow-up; most studies reported outcomes at 30 days, whereas some reported outcomes only to hospital discharge. The long-term risks and benefits of decreased antibiotic usage were not assessed. All of the studies were under-powered to address mortality. Of note, six of the 14 studies included significant funding from the manufacturer of the procalcitonin laboratory assay, and all were conducted in Europe, where baseline prescribing practices may be different than in the United States.
A study in Swiss primary care practices randomized adults with acute respiratory infections (n = 458) to treatment directed by a procalcitonin-guided algorithm vs. standard care.2 Patients in the procalcitonin group received antibiotics 72% less often than those who received standard care (95% CI, 66% to 78%). There was no difference in the risk of ongoing or relapsing symptoms at 28 days (OR = 1.0; 95% CI, 0.7 to 1.5) or days of restricted activity in the two weeks after randomization (8.7 vs. 8.6; mean difference = 0.1; 95% CI, −0.6 to 0.8). In a similar RCT of 550 patients presenting to German primary care clinics with acute respiratory tract infections, antibiotics were prescribed 41.6% less often in the procalcitonin algorithm group, with no difference between groups in the number of days of significant health impairment (9.04 vs. 9.00; mean difference = 0.04; 95% CI, −0.73 to 0.81).3
An RCT in Switzerland evaluated procalcitonin algorithms in 337 children (mean age = 3.8 years) with lower respiratory tract infections presenting to pediatric emergency departments.4 There was no difference in antibiotic prescription rates in the procalcitonin and control groups (OR = 1.26; 95% CI, 0.81 to 1.95), but there was a shorter mean duration of antibiotic use in the procalcitonin group (−1.8 days; 95% CI, −3.5 to −0.5).
Recommendations from Others
In 2012, the Agency for Healthcare Research and Quality conducted an independent systematic review and found that procalcitonin guidance reduced antibiotic prescription rates and the duration of antibiotic therapy without increasing morbidity or mortality.5 The review noted that most studies were conducted using quantitative procalcitonin assays, which take several hours to run and are not available at the point of care.
Copyright Family Physicians Inquiries Network. Used with permission.
