Am Fam Physician. 2017;95(5):online
Author disclosure: No relevant financial affiliations.

| Benefits | Harms |
|---|---|
| 1 in 4 using low-dose buprenorphine (2 to 6 mg) had retention in treatment | No study-related medication mortality was reported |
| 1 in 3 using medium-dose buprenorphine (7 to 16 mg) had retention in treatment | Uncertain adverse effects |
| 1 in 2 using high-dose buprenorphine (≥ 16 mg) had retention in treatment |
Details for This Review
Study Population: Adults with opioid dependence
Efficacy End Points: Treatment retention and illicit drug use suppression
Harm End Points: Mortality and adverse effects
Narrative: The United States is facing an opioid epidemic. Since 1999, overdose deaths involving opioids and heroin have quadrupled.1 Methadone, a full agonist, has traditionally been used to treat opioid and heroin dependence. Buprenorphine, a partial agonist, is an appealing alternative because it causes lower physical dependence, milder withdrawal symptoms, and is less likely to cause an overdose.2 This review examines the effectiveness of buprenorphine vs. placebo for retention in treatment and illicit opioid use, as measured by positive urinalysis or self-reported use.
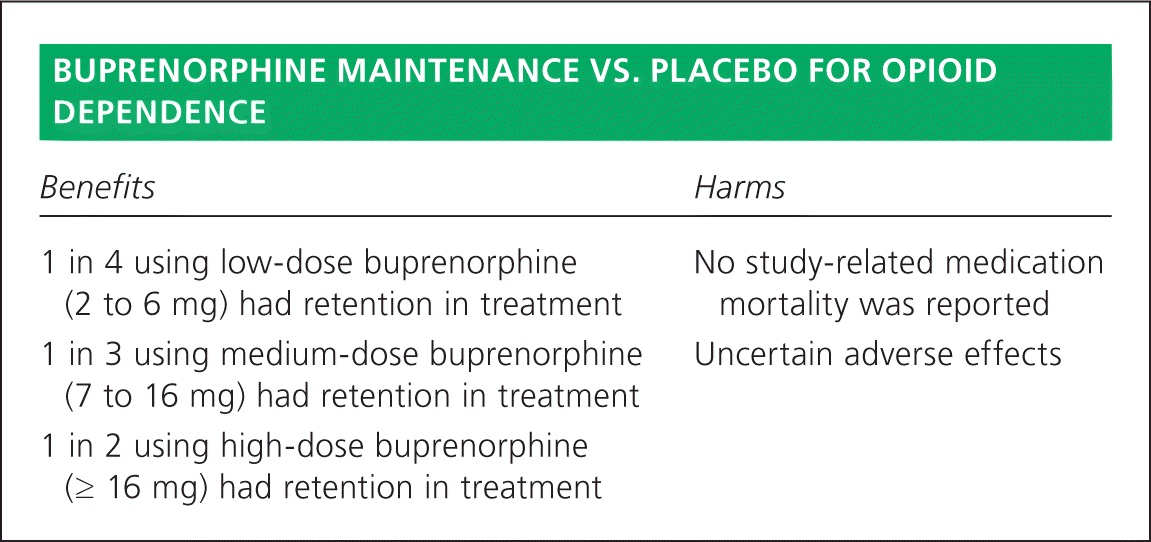
This Cochrane review included 31 studies with 5,430 participants, the majority of whom were male with an average age of 30 years.2 High-quality evidence shows that buprenorphine at all doses is more effective than placebo in retaining patients in treatment. An analysis of five studies using low-dose buprenorphine (2 to 6 mg) in 1,131 participants showed a benefit in treatment retention over placebo (relative risk [RR] = 1.5; 95% confidence interval [CI], 1.19 to 1.88). Medium-dose buprenorphine (7 to 16 mg) had slightly better patient retention in treatment (RR = 1.74; 95% CI, 1.06 to 2.87). High-dose buprenorphine (≥ 16 mg) was the most effective at retaining study participants in treatment (RR = 1.82; 95% CI, 1.15 to 2.90).
This review found moderate-quality evidence that buprenorphine is effective in suppressing illicit drug use, but only at doses of 16 mg or greater (three studies; 729 participants; standardized mean deviation [SMD] = −1.17; 95% CI, −1.85 to −0.49; number needed to treat = 2). Low-dose and medium-dose buprenorphine did not suppress illicit opioid use (SMD = 0.10; 95% CI, −0.80 to 1.01 and SMD = −0.08; 95% CI, −0.78 to 0.62, respectively).
Only five studies reported mortality data; no deaths were reported in three of the studies. In two of the studies there was a 20% mortality rate reported in the control group. Two deaths unconnected to the study medication were reported in one trial due to stab wounds and cancer.
Caveats: All trials included were randomized, controlled studies with low risk of bias. Twenty-two were double-blinded and 10 were open comparative trials. Of note, four of the studies used a 1-mg dose of buprenorphine as the placebo. The authors note that this is unlikely to bias results given the clinically insignificant dose. The included studies varied in their reporting of adverse events, if they reported them at all.
Current treatment of opioid dependence generally uses an individualized dosing schedule; therefore, flexible-dose methadone or flexible-dose buprenorphine may be more clinically relevant. This review also compared 11 studies with 1,391 participants receiving either flexible-dose methadone or flexible-dose buprenorphine. The methadone group was more likely to be retained in treatment (RR = 0.83; 95% CI, 0.73 to 0.95). There was no difference between the two groups with regard to illicit opioid use (SMD = −0.11; 95% CI, −0.23 to 0.02).
Conclusion: Based on current evidence, buprenorphine is effective in retaining patients in treatment at all doses; however, only doses of at least 16 mg prevent illicit drug use during therapy. Studies using flexible-dose buprenorphine without using buprenorphine as a placebo may be more clinically relevant to actual practice. The harms from treatment are currently unclear because of poor reporting of adverse events.
The views expressed are those of the author and do not reflect the official policy or position of the Bayne-Jones Army Community Hospital, the Army Medical Department, the Department of Defense, or the U.S. government.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Contributing Editor, and Daniel Runde, MD, from the NNT Group (theNNT.com).
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
This review is available from the NNT Group at http://www.thennt.com/nnt/buprenorphine-maintenance-vs-placebo-opioid-dependence/.