
Am Fam Physician. 2017;95(9):online
Author disclosure: No relevant financial affiliations.

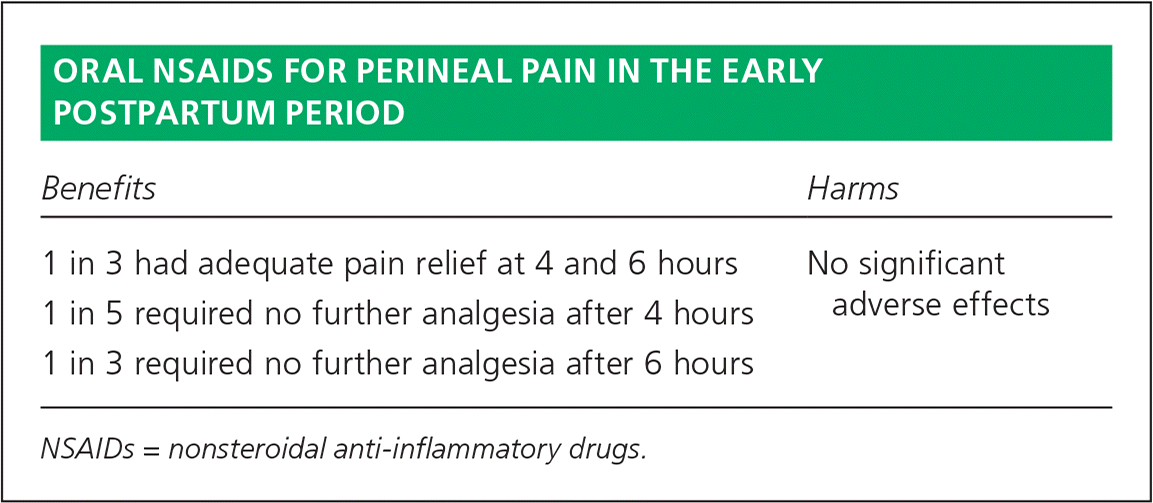
| Benefits | Harms |
|---|---|
| 1 in 3 had adequate pain relief at 4 and 6 hours | No significant adverse effects |
| 1 in 5 required no further analgesia after 4 hours | |
| 1 in 3 required no further analgesia after 6 hours |
Details for This Review
Study Population: Postpartum women who had perineal pain after vaginal delivery and were not breastfeeding
Efficacy End Points: Adequate pain relief defined by at least 50% reduction in pain at four and six hours; no additional need for analgesics
Harm End Points: Adverse effects at four and six hours, including nausea, vomiting, sedation, constipation, diarrhea, drowsiness, sleepiness, and psychological impacts
Narrative: Perineal pain is common after vaginal childbirth. Physicians delivering newborns should be comfortable treating this pain. Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used in treating postpartum pain and have been studied extensively.1 These medications are not completely benign because they have been associated with adverse cardiovascular, gastrointestinal, and renal side effects.2 With these potential adverse effects in mind, this review evaluated oral NSAIDs for the treatment of perineal pain in the early postpartum period in women who have trauma to the perineum from first- to fourth-degree lacerations.
This review included 28 studies that examined 13 NSAIDs and included 4,181 postpartum women. A single-dose NSAID was given to 2,642 women in the early post-partum period, whereas 1,539 received placebo. More women who received an NSAID had adequate pain relief in the first four hours and the first six hours following the intervention compared with those receiving a placebo. Adequate pain relief was defined as at least 50% relief in pain by subjective report or by using the Total Pain Relief score or the Summed Pain Intensity Difference score. Compared with women receiving placebo, those receiving an NSAID achieved adequate pain relief in four hours (relative risk [RR] = 1.91; 95% confidence interval [CI], 1.64 to 2.23) and in six hours (RR = 1.92; 95% CI, 1.69 to 2.17). The numbers needed to treat to prevent use of additional analgesics at four and six hours were 5 and 3, respectively. Women receiving an NSAID had less need for additional analgesia at four hours compared with placebo (RR = 0.39; 95% CI, 0.26 to 0.58) and six hours (RR = 0.32; 95% CI, 0.26 to 0.40). Only one study recorded adverse events in the four-hour post-intervention group with very small numbers. Compared with placebo, those receiving an NSAID had no difference in overall adverse events (RR = 1.38; 95% CI, 0.71 to 2.70). These adverse events included nausea, vomiting, sedation, constipation, diarrhea, drowsiness, sleepiness, and psychological impacts.
Caveats: This review included only randomized controlled trials. Most of the studies were completed in the 1980s and had a wide range of sample sizes, patient types, and treatment settings. The individual studies had poor reporting of bias, making it difficult to ascertain the level of bias. As a result, the quality of evidence in the individual studies ranged from very low to low grades.
Neonatal outcomes were not evaluated in any of the studies. Finally, including non-breastfeeding women could be considered a limitation as well. The authors of the Cochrane review could not identify a physiologic reason to exclude them from the study, but assumed they were excluded because many of the studies were published in the 1980s before much of the evidence supporting breastfeeding was discovered and published. There is no clear reason to think these results do not apply to breast-feeding mothers as well.
