Am Fam Physician. 2017;95(11):online
Author disclosure: No relevant financial affiliations.

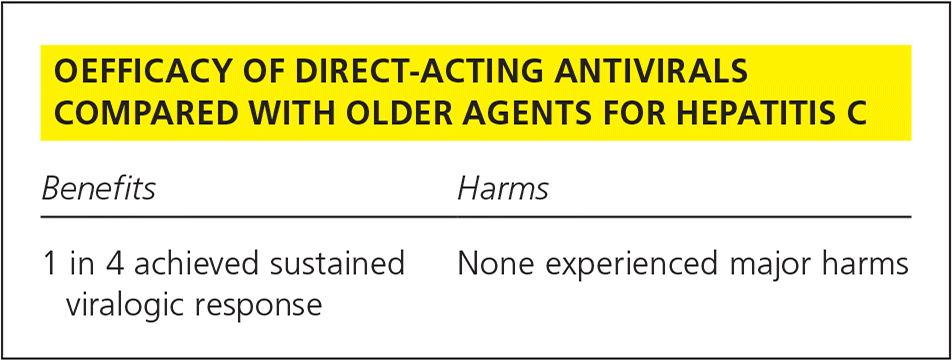
| Benefits | Harms |
|---|---|
| 1 in 4 achieved sustained viralogic response | None experienced major harms |
Details for This Review
Study Population: Treatment-naive patients with hepatitis C virus genotype 1
Efficacy End Points: Sustained viralogic response (SVR) at 24 weeks (absence of detectable virus in the blood)
Harm End Points: Anemia, fatigue, death, hospital admission
Narrative: Hepatitis C virus is typically latent (i.e., silent) for decades; therefore, 80% to 85% of persons infected will not experience illness caused by the infection. Conversely, 15% to 20% of patients with untreated hepatitis will eventually develop liver failure or cancer.1 Standard treatment to prevent these developments includes interferon and ribavirin (Rebetol), a difficult-to-tolerate regimen that achieves SVR (i.e., the absence of virus in the blood) in approximately 50% of patients but is commonly associated with anemia and fatigue. The use of newer direct-acting antiviral agents may improve SVR rate and tolerability.2
The review summarized here addresses all major trials of second-generation direct-acting antiviral agents. To better understand the potentially unique benefits of this class, we focus on trials comparing any direct-acting antiviral-based regimen with interferon plus ribavirin. Despite 16 trials being identified, only five (all using simeprevir [Olysio]) were similar enough to be pooled. Simeprevir, when added to interferon and ribavirin, improved SVR without increasing adverse effects: 82% vs. 56% with the older regimen, a 26% absolute improvement (number needed to treat = 4).
Although pooled data for other agents such as daclatasvir (Daklinza, used in combination with sofosbuvir [Sovaldi]), ledipasvir (used in combination with sofosbuvir [Harvoni]), sofosbuvir, and paritaprevir (used in combination with ombitasvir and ritonavir [Norvir]) were unavailable (because of their indications to be used in combination with other agents), indirect comparisons suggest similar rates of SVR, typically within 5% to 10%.
Caveats: These data are promising for direct-acting antivirals, and suggest that similar patients have an 80% to 90% chance of SVR when receiving them. This cannot tell us whether these agents prevent liver cancer or liver failure, the goals patients care about, because it is unknown if SVR, a surrogate marker, is adequate to prevent these outcomes or, if so, how consistently. Even assuming SVR leads to universal prevention, the numbers are limited: Up to 20% of infected patients can potentially benefit, and 26% more can achieve SVR with direct-acting antiviral use; therefore, at best, approximately one in 20 patients (0.20 × 0.26 = .05) who receive these antivirals would benefit.
Patients with hepatitis C typically present for care because of symptoms or illness. With increased screening, treatment is now commonly considered in otherwise healthy persons who may be at even lower risk of liver problems. Assuming a modest reduction to 10% of untreated patients who would progress to liver failure or cancer, even an overall 82% SVR rate would mean roughly 8% of patients benefit. Comparing this with the 5% to 8% rate of serious adverse events occurring with any treatment regimen suggests that direct-acting antiviral agents—or any other hepatitis C treatment—deserve close examination and shared decision making.3
A recent U.S. Food and Drug Administration report warns about the risk of reactivating hepatitis B virus in patients with current or previous infection who are treated with certain direct-acting antivirals for hepatitis C virus. In a few cases, hepatitis B virus reactivation in patients treated with direct-acting antivirals resulted in serious liver problems or death. Hepatitis B virus reactivation usually occurred within four to eight weeks.4
The studies in this review are relevant only to patients with hepatitis C who have not been treated and do not have cirrhosis. Furthermore, we focused on data from a single agent, simeprevir. Pooled estimates of other direct-acting antivirals were based on “network” meta-analysis, a technique that combines results from a variety of comparisons and settings. This indirect method may be helpful in the absence of head-to-head trials, but we believe the results are less reliable. Network meta-analysis and data from other investigators suggest slightly better results with sofosbuvir.5
Direct-acting antivirals may have introduced a new era in drug pricing. Sofosbuvir, for example, costs $168,000 for a 24-week course, presenting potential barriers to access that have spurred new debates about value and resource allocation. Medicaid reimbursement for sofosbuvir varies, and some states require pretreatment drug screening, abstinence from drugs and alcohol, or suppressed human immunodeficiency virus status, making access a complicated and ongoing issue.6
Direct-acting antivirals for hepatitis C treatment are classified as yellow because more data will be required to determine whether they offer true patient benefit. In particular, follow-up reports on liver cancer and liver failure, as well as trials of hepatitis C screening, are needed. In the meantime, decisions about direct-acting antivirals require thoughtful consideration of benefits and harms, cost-utility, and the limits of financial resources.