Am Fam Physician. 2017;96(10):656-663
NOTE: On October 25, 2017, the Centers for Disease Control and Prevention's (CDC's) Advisory Committee on Immunization Practices voted to recommend the herpes zoster recombinant subunit vaccine (Shingrix) for healthy adults 50 years and older, including those who previously received Zostavax, to prevent shingles and related complications. This article has been revised to reflect the new recommendations. For more information, visit the CDC page on shingles (herpes zoster) vaccination.
Author disclosure: No relevant financial affiliations.
Herpes zoster, or shingles, is caused by reactivation of varicella zoster virus, which causes chickenpox. There are an estimated 1 million cases in the Unites States annually, with an individual lifetime risk of 30%. Patients with conditions that decrease cell-mediated immunity are 20 to 100 times more likely to develop herpes zoster. Patients may present with malaise, headache, low-grade fever, and abnormal skin sensations for two to three days before the classic maculopapular rash appears. The rash is usually unilateral, confined to a single dermatome, and typically progresses to clear vesicles that become cloudy and crust over in seven to 10 days. Herpes zoster can be treated with acyclovir, valacyclovir, or famciclovir, ideally within 72 hours of the development of the rash. Postherpetic neuralgia is the most common complication, occurring in about one in five patients. It is defined as pain in a dermatomal distribution sustained for at least 90 days after acute herpes zoster. Treatment is focused on symptom control and includes topical lidocaine or capsaicin and oral gabapentin, pregabalin, or tricyclic antidepressants. Two varicella zoster virus vaccines decrease the incidence of herpes zoster and are approved for adults 50 years and older. The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices recommends two doses of adjuvant recombinant varicella zoster virus accine for adults 50 years and older, including those who have already had the live varicella zoster virus vaccine.
Herpes zoster, or shingles, is caused by reactivation of varicella zoster virus (VZV), which causes chickenpox. It presents as painful blistering and occurs when VZV cell-mediated immunity wanes with age or immunocompromise.1,2 Herpes zoster may be associated with acute pain; postherpetic neuralgia; and visual, neurologic, or visceral complications.3,4
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Although herpes zoster typically is diagnosed clinically, if laboratory confirmation is needed, polymerase chain reaction testing of vesicle or other fluids is preferred for diagnosis because of its high sensitivity (95%) and specificity (100%). | C | 7 |
| Acyclovir, valacyclovir (Valtrex), and famciclovir are effective treatments for herpes zoster and ideally should be started within 72 hours of the appearance of the rash to decrease the duration of symptoms and severity of pain. | B | 1, 2, 7, 14–16 |
| Capsaicin 8% patches, applied for 30 to 90 minutes, provide effective pain relief for patients with postherpetic neuralgia. | A | 40 |
| Gabapentin (Neurontin) and pregabalin (Lyrica) can be used for treatment of postherpetic neuralgia. | A | 42 |
| Amitriptyline, nortriptyline (Pamelor), and desipramine can be used for pain relief in patients with postherpetic neuralgia (number needed to treat = 3; 95% confidence interval, 2 to 4). | A | 26, 44 |
| The adjuvant recombinant varicella zoster virus vaccine (Shingrix) should be given to patients 50 years and older, including those who have already had the live varicella virus vaccine (Zostavax). | B | 48, 49 |
Epidemiology
An estimated 1 million cases of herpes zoster occur in the Unites States annually, with an individual lifetime risk of 30%.5 About 2% to 3% of patients with this condition are hospitalized each year, with costs ranging from $1 billion to $2 billion annually.4 In a typical family practice with 1,500 patients, three to five cases of herpes zoster can be expected each year.2
Almost all adults in the United States have been exposed to VZV.1,6 The incidence of herpes zoster ranges from one to three cases per 1,000 person-years in those younger than 50 years. Age is a major risk factor; T lymphocyte–specific immunity to the virus wanes over time, and more than one-half of unvaccinated patients 85 years and older will be affected.3,7 Women are at increased risk, whereas blacks are at decreased risk.5,7 Patients with conditions that decrease cell-mediated immunity (e.g., lymphoproliferative disorders, immunosuppressive drug use, human immunodeficiency virus sero-positivity) are at 20 to 100 times greater risk compared with age-matched controls.2
Clinical Presentation
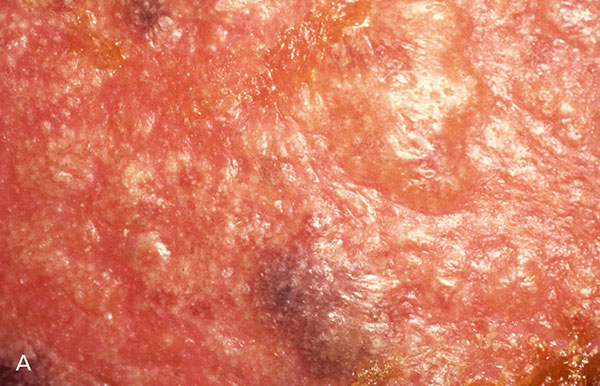
As the initial VZV infection resolves, viral particles travel to cranial and dorsal root ganglia, where they are shielded from blood antibodies. The virus remains latent and may reactivate as cell-mediated immunity wanes.3,8 Not all of the contributing factors are understood, but under the right conditions, the virus replicates, causing a nonspecific prodrome with malaise, headache, fever, or abnormal skin sensations (e.g., itching, burning, pain). The classic rash typically appears after two to three days, with new lesions appearing over three to five days (Figure 1). Usually affecting a single, unilateral dermatome, maculopapular lesions appear proximally to distally, progressing to clear vesicles that become cloudy and eventually crust over in seven to 10 days. Lesions usually heal two to four weeks after onset, but scarring and pigmentation changes are common.7
Diagnosis
The diagnosis of herpes zoster is typically clinical. Although herpes zoster is difficult to identify during the prodrome, the appearance of the typical exanthem aids in diagnosis. Testing is typically not needed, but it may be considered in patients with recurring lesions that are suspicious for herpes simplex, or in those with suspected zoster sine herpete, in which the virus causes pain without lesions. Testing may also be considered in atypical presentations, such as the widely disseminated lesions that may occur in immunocompromised patients.7 Testing is also helpful in differentiating herpes zoster from other vesicular dermatoses, such as contact dermatitis and dermatitis herpetiformis.2 Polymerase chain reaction testing of vesicle or other body fluids is preferred because of its high sensitivity and specificity (95% and 100%, respectively) and short turnaround (typically one day).1,7
Management of Acute Herpes Zoster
Herpes zoster is treated with oral guanosine analogues (Table 11,10–13 ). These medications target VZV by relying on viral kinases for phosphorylation, which promotes incorporation into viral DNA, thus disrupting replication.1 Acyclovir is less expensive but has lower bioavailability and must be taken five times per day. Valacyclovir (Valtrex), a pro-drug of acyclovir, is taken three times per day, as is famciclovir.10 Acyclovir is the only antiviral medication approved for the treatment of herpes zoster in children. Patients with severe disease, especially those with immunocompromise, should be treated with intravenous acyclovir.1 Although treatment of herpes zoster ideally should be started within 72 hours of the appearance of the rash,1,2,7 treatment is still warranted outside the 72-hour window if new skin lesions are developing or if ophthalmic or neurologic complications are present.7
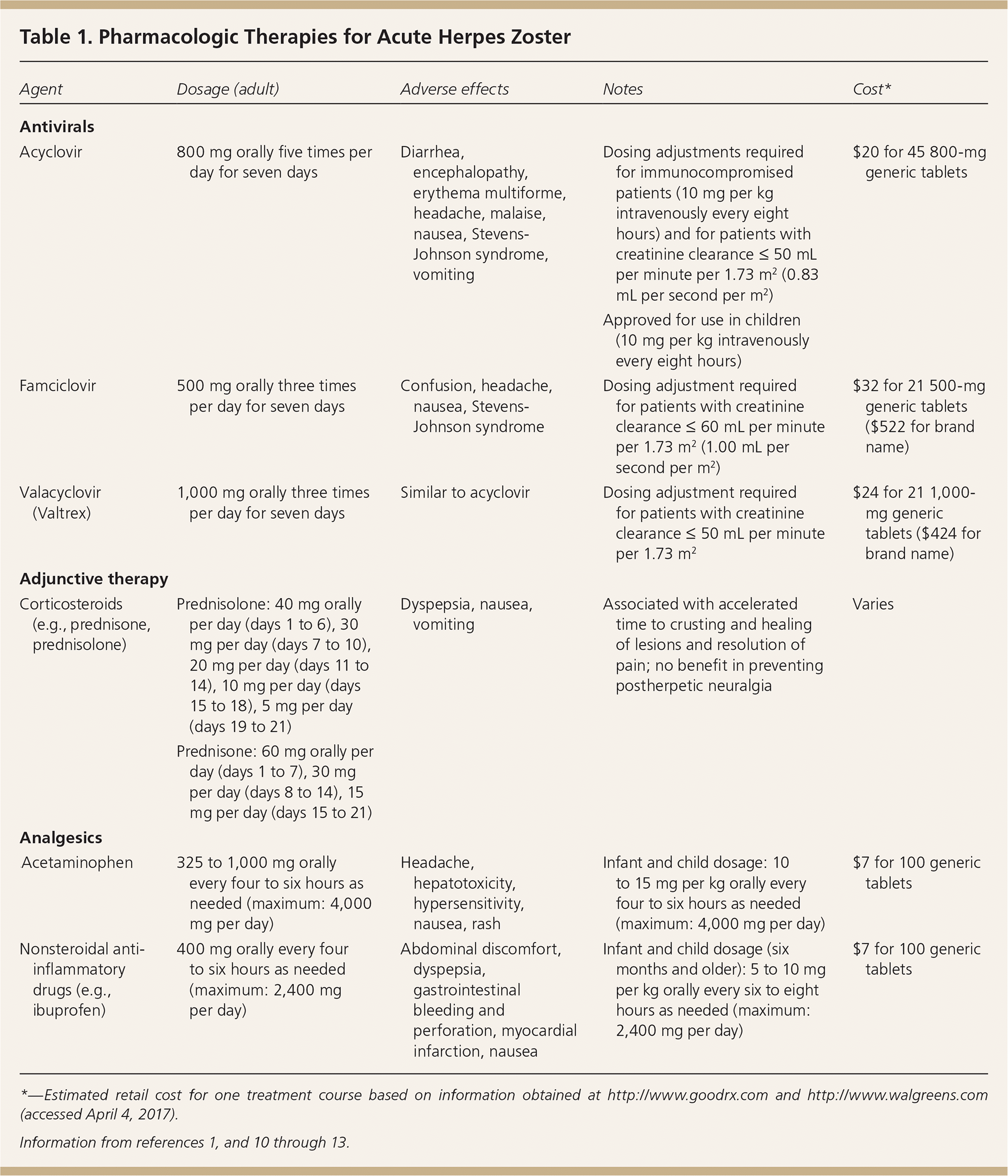
| Agent | Dosage (adult) | Adverse effects | Notes | Cost* |
|---|---|---|---|---|
| Antivirals | ||||
| Acyclovir | 800 mg orally five times per day for seven days | Diarrhea, encephalopathy, erythema multiforme, headache, malaise, nausea, Stevens-Johnson syndrome, vomiting | Dosing adjustments required for immunocompromised patients (10 mg per kg intravenously every eight hours) and for patients with creatinine clearance ≤ 50 mL per minute per 1.73 m2 (0.83 mL per second per m2) | $20 for 45 800-mg generic tablets |
| Approved for use in children (10 mg per kg intravenously every eight hours) | ||||
| Famciclovir | 500 mg orally three times per day for seven days | Confusion, headache, nausea, Stevens-Johnson syndrome | Dosing adjustment required for patients with creatinine clearance ≤ 60 mL per minute per 1.73 m2 (1.00 mL per second per m2) | $32 for 21 500-mg generic tablets ($522 for brand name) |
| Valacyclovir (Valtrex) | 1,000 mg orally three times per day for seven days | Similar to acyclovir | Dosing adjustment required for patients with creatinine clearance ≤ 50 mL per minute per 1.73 m2 | $24 for 21 1,000-mg generic tablets ($424 for brand name) |
| Adjunctive therapy | ||||
| Corticosteroids (e.g., prednisone, prednisolone) | Prednisolone: 40 mg orally per day (days 1 to 6), 30 mg per day (days 7 to 10), 20 mg per day (days 11 to 14), 10 mg per day (days 15 to 18), 5 mg per day (days 19 to 21) | Dyspepsia, nausea, vomiting | Associated with accelerated time to crusting and healing of lesions and resolution of pain; no benefit in preventing postherpetic neuralgia | Varies |
| Prednisone: 60 mg orally per day (days 1 to 7), 30 mg per day (days 8 to 14), 15 mg per day (days 15 to 21) | ||||
| Analgesics | ||||
| Acetaminophen | 325 to 1,000 mg orally every four to six hours as needed (maximum: 4,000 mg per day) | Headache, hepatotoxicity, hypersensitivity, nausea, rash | Infant and child dosage: 10 to 15 mg per kg orally every four to six hours as needed (maximum: 4,000 mg per day) | $7 for 100 generic tablets |
| Nonsteroidal anti-inflammatory drugs (e.g., ibuprofen) | 400 mg orally every four to six hours as needed (maximum: 2,400 mg per day) | Abdominal discomfort, dyspepsia, gastrointestinal bleeding and perforation, myocardial infarction, nausea | Infant and child dosage (six months and older): 5 to 10 mg per kg orally every six to eight hours as needed (maximum: 2,400 mg per day) | $7 for 100 generic tablets |
Glucocorticoids are an adjunct to antiviral therapy; they reduce acute pain and promote early healing.12,13 Glucocorticoids do not reduce the incidence of postherpetic neuralgia18 and should not be used without antivirals. Treatment of acute pain depends on its severity and impact.19 Mild to moderate pain may be controlled with acetaminophen or nonsteroidal anti-inflammatory agents. Severe cases may require opioids, although such prescriptions should follow established guidelines.7,20–22 Anticonvulsants, tricyclic antidepressants, or nerve blocks may be considered for patients with suboptimal pain control.7,21,23–26
Postherpetic Neuralgia
Postherpetic neuralgia, the most common complication of herpes zoster, is defined as pain in a dermatomal distribution that is sustained for at least 90 days after the rash. It occurs in approximately 20% of patients with herpes zoster,27,28 and 80% of cases occur in patients 50 years or older.29 Pain is described as burning or electric shock–like and may be associated with allodynia or hyperalgesia.27 Postherpetic neuralgia is caused by nerve damage secondary to an inflammatory response induced by viral replication within a nerve.27,30 Risk factors include older age, severe prodrome or rash, severe acute zoster pain, ophthalmic involvement, immunosuppression, and chronic conditions such as diabetes mellitus and lupus.2,31–33 Pain from postherpetic neuralgia is often debilitating and affects physical functioning, psychological well-being, and quality of life.34 Pain-management strategies should focus on symptom control. Although some patients have complete resolution of symptoms at several years, others continue medications indefinitely 27,35 (Table 211,27,36,37 ).
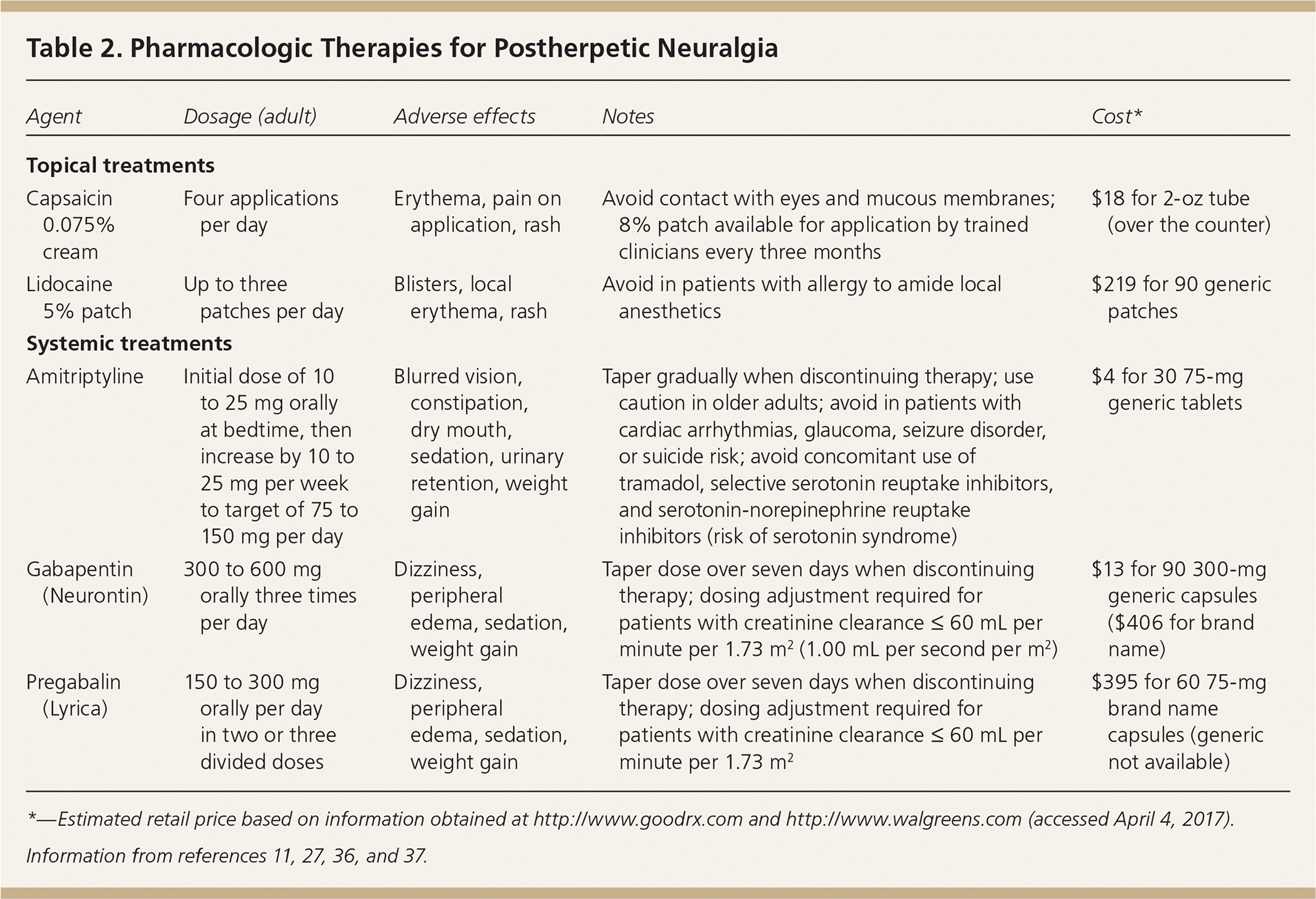
| Agent | Dosage (adult) | Adverse effects | Notes | Cost* |
|---|---|---|---|---|
| Topical treatments | ||||
| Capsaicin 0.075% cream | Four applications per day | Erythema, pain on application, rash | Avoid contact with eyes and mucous membranes; 8% patch available for application by trained clinicians every three months | $18 for 2-oz tube (over the counter) |
| Lidocaine 5% patch | Up to three patches per day | Blisters, local erythema, rash | Avoid in patients with allergy to amide local anesthetics | $219 for 90 generic patches |
| Systemic treatments | ||||
| Amitriptyline | Initial dose of 10 to 25 mg orally at bedtime, then increase by 10 to 25 mg per week to target of 75 to 150 mg per day | Blurred vision, constipation, dry mouth, sedation, urinary retention, weight gain | Taper gradually when discontinuing therapy; use caution in older adults; avoid in patients with cardiac arrhythmias, glaucoma, seizure disorder, or suicide risk; avoid concomitant use of tramadol, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors (risk of serotonin syndrome) | $4 for 30 75-mg generic tablets |
| Gabapentin (Neurontin) | 300 to 600 mg orally three times per day | Dizziness, peripheral edema, sedation, weight gain | Taper dose over seven days when discontinuing therapy; dosing adjustment required for patients with creatinine clearance ≤ 60 mL per minute per 1.73 m2 (1.00 mL per second per m2) | $13 for 90 300-mg generic capsules ($406 for brand name) |
| Pregabalin (Lyrica) | 150 to 300 mg orally per day in two or three divided doses | Dizziness, peripheral edema, sedation, weight gain | Taper dose over seven days when discontinuing therapy; dosing adjustment required for patients with creatinine clearance ≤ 60 mL per minute per 1.73 m2 | $395 for 60 75-mg brand name capsules (generic not available) |
TOPICAL TREATMENTS
There are two topical preparations approved for management of postherpetic neuralgia. The lidocaine 5% patch has a favorable adverse effect profile and is considered first-line therapy despite limited evidence of effectiveness. Although one systematic review demonstrated improved pain,38 a Cochrane review of six randomized controlled trials (RCTs) concluded the evidence supporting its use is lacking.39
Capsaicin is also an option for pain relief. A meta-analysis of four RCTs with 1,272 patients concluded that capsaicin 8% patches applied for 30 to 90 minutes provided greater pain relief than low-concentration topical capsaicin after 12 weeks (number needed to treat [NNT] = 7; 95% confidence interval [CI], 5 to 15).40 However, the 8% patch is irritating and likely to cause pain when applied. A trained clinician should pretreat the application site with topical anesthetic before affixing the patch.28 Lower-potency capsaicin cream (0.075%) has also been used to treat postherpetic neuralgia, although a Cochrane review concluded that there was insufficient evidence to recommend its use.41
SYSTEMIC TREATMENTS
The anticonvulsants gabapentin (Neurontin) and pregabalin (Lyrica) are approved for treatment of postherpetic neuralgia. Several meta-analyses have shown that gabapentin (1,800 to 3,600 mg per day; NNT = 8; 95% CI, 5 to 14) and pregabalin (600 mg per day; NNT = 4; 95% CI, 3 to 9) were more effective than placebo in achieving 50% reduction in pain.42 Despite their effectiveness, the time needed to titrate these agents to an effective dose (up to 10 weeks) and their adverse effects (e.g., somnolence) may limit their use.43
Tricyclic antidepressants are also effective in treating postherpetic neuralgia. A meta-analysis of four RCTs comparing amitriptyline, nortriptyline (Pamelor), and desipramine with placebo estimated an NNT of 3 (95% CI, 2 to 4) to achieve meaningful pain relief.44 A Cochrane review found no differences in pain relief among the tricyclic antidepressants after four weeks, but all were superior to placebo.26 Up to one-fourth of patients taking tricyclic antidepressants discontinue treatment because of adverse effects such as confusion, sedation, urinary retention, and cardiotoxicity.26,28
Opioids are considered third-line treatment for postherpetic neuralgia. A Cochrane review concluded that the benefit of opioids for neuropathic pain is uncertain because of a lack of unbiased evidence.45 Two systematic reviews found that tramadol provided significant pain relief in patients with postherpetic neuralgia (NNT = 4 or 5).44,46
The potential harms of systemic therapies for postherpetic neuralgia should be considered before treating older patients or those with comorbidities. A thorough assessment, including a medication review and physical examination focusing on balance, gait, and orthostatic vital signs, will help minimize adverse effects of treatment and interactions between treatments and other medications.30 The American Geriatrics Society advocates initiating medications for persistent pain at low doses and titrating slowly.47
Prevention
[Updated 11/16/17]
Herpes zoster and postherpetic neuralgia are vaccine preventable. On October 23, 2017, the U.S. Food and Drug Administration approved an adjuvant recombinant VZV vaccine (Shingrix) for the prevention of shingles.48 The incidence of herpes zoster in those receiving the vaccine decreased by 96% (95% CI, 90% to 98%) compared with placebo.49 It is well tolerated, and because its effectiveness is not age dependent and does not carry the risk of inducing herpes zoster,49 it has been recommended by the Advisory Committee on Immunization Practices as the preferred method of preventing herpes zoster and postherpetic nerualgia. The vaccine is recommended for adults 50 years and older, including those who have already had the live VZV vaccine (Zostavax). It is administered in two doses, with the second dose given two to six months after the first.48
Before the advent of the recombinant VZV vaccine, live VZV vaccine was the recommended immunization, approved for adults 50 years and older. The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices had recommended vaccination for adults 60 years and older, regardless of whether they had naturally occurring varicella.50 The live VZV vaccine is contraindicated in immunosuppressed persons, those with human immunodeficiency virus infection and CD4 lymphocyte counts less than 200 per mm3 (0.20 × 109 per L), patients undergoing cancer treatment, and those with cancer affecting the bones or lymphatic system. Vaccine effectiveness is 69% in the first year, but wanes to 4% in the eighth year51; there are no recommendations for revaccinating persons who receive the vaccine at 60 years or older.
Although the live VZV vaccine is effective, it is underused, likely in part because of the cost. Cost is likely to be a factor in the uptake of the new vaccine, especially because it is given in two doses instead of one. In 2013, the VZV vaccination rate was only 24.2% among adults 60 years and older.52 White adults receive the vaccine at almost three times the rate of blacks and Hispanics.52 Patient education can increase vaccination rates by helping patients understand the benefits and ways in which patients may be able to work with insurance companies to find an affordable means of obtaining it.
Data Sources: We searched the Cochrane Database of Systematic Reviews, the National Guideline Clearing-house, PubMed, and Essential Evidence Plus using the following key words: herpes zoster, varicella zoster virus, postherpetic neuralgia, and zoster vaccine. Search dates: October 2015 through March 2017.
Figure 1 courtesy of Leonard Sperling, MD, Colonel, U.S. Army (retired).
The views expressed in this article are the authors' own and do not necessarily reflect the views of the U.S. Army, U.S. Navy, U.S. Air Force, the Department of Defense, or the U.S. government.