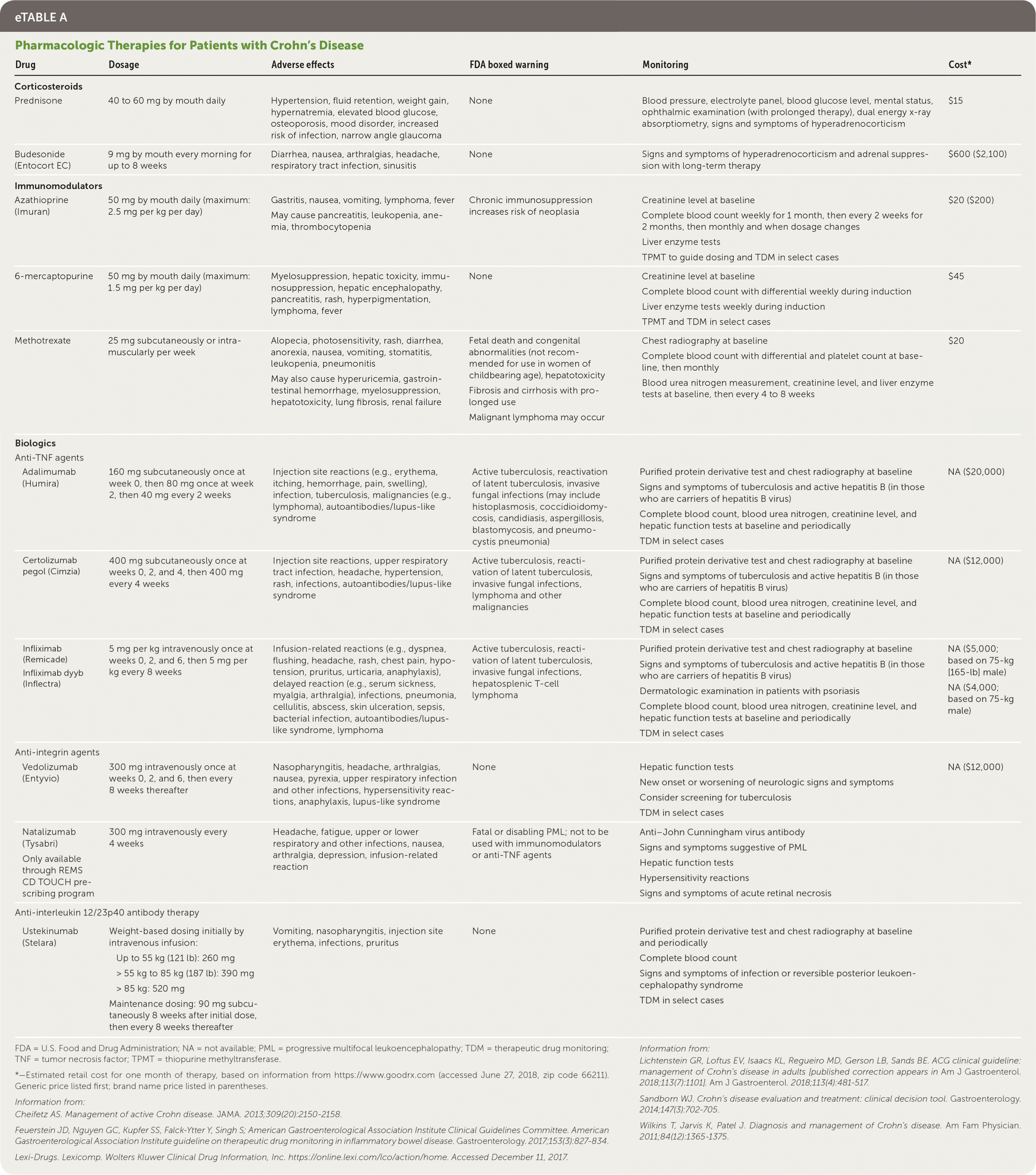
| Drug | Dosage | Adverse effects | FDA boxed warning | Monitoring | Cost* |
|---|---|---|---|---|---|
| Corticosteroids | |||||
| Prednisone | 40 to 60 mg by mouth daily | Hypertension, fluid retention, weight gain, hypernatremia, elevated blood glucose, osteoporosis, mood disorder, increased risk of infection, narrow angle glaucoma | None | Blood pressure, electrolyte panel, blood glucose level, mental status, ophthalmic examination (with prolonged therapy), dual energy x-ray absorptiometry, signs and symptoms of hyperadrenocorticism | $15 |
| Budesonide (Entocort EC) | 9 mg by mouth every morning for up to 8 weeks | Diarrhea, nausea, arthralgias, headache, respiratory tract infection, sinusitis | None | Signs and symptoms of hyperadrenocorticism and adrenal suppression with long-term therapy | $600 ($2,100) |
| Immunomodulators | |||||
| Azathioprine (Imuran) | 50 mg by mouth daily (maximum: 2.5 mg per kg per day) | Gastritis, nausea, vomiting, lymphoma, fever May cause pancreatitis, leukopenia, anemia, thrombocytopenia | Chronic immunosuppression increases risk of neoplasia | Creatinine level at baseline Complete blood count weekly for 1 month, then every 2 weeks for 2 months, then monthly and when dosage changes Liver enzyme tests TPMT to guide dosing and TDM in select cases | $20 ($200) |
| 6-mercaptopurine | 50 mg by mouth daily (maximum: 1.5 mg per kg per day) | Myelosuppression, hepatic toxicity, immunosuppression, hepatic encephalopathy, pancreatitis, rash, hyperpigmentation, lymphoma, fever | None | Creatinine level at baseline Complete blood count with differential weekly during induction Liver enzyme tests weekly during induction TPMT and TDM in select cases | $45 |
| Methotrexate | 25 mg subcutaneously or intramuscularly per week | Alopecia, photosensitivity, rash, diarrhea, anorexia, nausea, vomiting, stomatitis, leukopenia, pneumonitis May also cause hyperuricemia, gastrointestinal hemorrhage, myelosuppression, hepatotoxicity, lung fibrosis, renal failure | Fetal death and congenital abnormalities (not recommended for use in women of childbearing age), hepatotoxicity Fibrosis and cirrhosis with prolonged use Malignant lymphoma may occur | Chest radiography at baseline Complete blood count with differential and platelet count at baseline, then monthly Blood urea nitrogen measurement, creatinine level, and liver enzyme tests at baseline, then every 4 to 8 weeks | $20 |
| Biologics | |||||
| Anti-TNF agents | |||||
| Adalimumab (Humira) | 160 mg subcutaneously once at week 0, then 80 mg once at week 2, then 40 mg every 2 weeks | Injection site reactions (e.g., erythema, itching, hemorrhage, pain, swelling), infection, tuberculosis, malignancies (e.g., lymphoma), autoantibodies/lupus-like syndrome | Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections (may include histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystis pneumonia) | Purified protein derivative test and chest radiography at baseline Signs and symptoms of tuberculosis and active hepatitis B (in those who are carriers of hepatitis B virus) Complete blood count, blood urea nitrogen, creatinine level, and hepatic function tests at baseline and periodically TDM in select cases | NA ($20,000) |
| Certolizumab pegol (Cimzia) | 400 mg subcutaneously once at weeks 0, 2, and 4, then 400 mg every 4 weeks | Injection site reactions, upper respiratory tract infection, headache, hypertension, rash, infections, autoantibodies/lupus-like syndrome | Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections, lymphoma and other malignancies | Purified protein derivative test and chest radiography at baseline Signs and symptoms of tuberculosis and active hepatitis B (in those who are carriers of hepatitis B virus) Complete blood count, blood urea nitrogen, creatinine level, and hepatic function tests at baseline and periodically TDM in select cases | NA ($12,000) |
| Infliximab (Remicade) Infliximab dyyb (Inflectra) | 5 mg per kg intravenously once at weeks 0, 2, and 6, then 5 mg per kg every 8 weeks | Infusion-related reactions (e.g., dyspnea, flushing, headache, rash, chest pain, hypotension, pruritus, urticaria, anaphylaxis), delayed reaction (e.g., serum sickness, myalgia, arthralgia), infections, pneumonia, cellulitis, abscess, skin ulceration, sepsis, bacterial infection, autoantibodies/lupus-like syndrome, lymphoma | Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections, hepatosplenic T-cell lymphoma | Purified protein derivative test and chest radiography at baseline Signs and symptoms of tuberculosis and active hepatitis B (in those who are carriers of hepatitis B virus) Dermatologic examination in patients with psoriasis Complete blood count, blood urea nitrogen, creatinine level, and hepatic function tests at baseline and periodically TDM in select cases | NA ($5,000; based on 75-kg [165-lb] male) NA ($4,000; based on 75-kg male) |
| Anti-integrin agents | |||||
| Vedolizumab (Entyvio) | 300 mg intravenously once at weeks 0, 2, and 6, then every 8 weeks thereafter | Nasopharyngitis, headache, arthralgias, nausea, pyrexia, upper respiratory infection and other infections, hypersensitivity reactions, anaphylaxis, lupus-like syndrome | None | Hepatic function tests New onset or worsening of neurologic signs and symptoms Consider screening for tuberculosis TDM in select cases | NA ($12,000) |
| Natalizumab (Tysabri) Only available through REMS CD TOUCH prescribing program | 300 mg intravenously every 4 weeks | Headache, fatigue, upper or lower respiratory and other infections, nausea, arthralgia, depression, infusion-related reaction | Fatal or disabling PML; not to be used with immunomodulators or anti-TNF agents | Anti–John Cunningham virus antibody Signs and symptoms suggestive of PML Hepatic function tests Hypersensitivity reactions Signs and symptoms of acute retinal necrosis | |
| Anti-interleukin 12/23p40 antibody therapy | |||||
| Ustekinumab (Stelara) | Weight-based dosing initially by intravenous infusion Up to 55 kg (121 lb): 260 mg > 55 kg to 85 kg (187 lb): 390 mg > 85 kg: 520 mg Maintenance dosing: 90 mg subcutaneously 8 weeks after initial dose, then every 8 weeks thereafter | Vomiting, nasopharyngitis, injection site erythema, infections, pruritus | None | Purified protein derivative test and chest radiography at baseline and periodically Complete blood count Signs and symptoms of infection or reversible posterior leukoencephalopathy syndrome TDM in select cases | |