Am Fam Physician. 2019;100(6):372-373
Author disclosure: No relevant financial affiliations.
Glycopyrronium (Qbrexza) topical wipes are labeled for the treatment of hyperhidrosis. Glycopyrronium wipes contain a topically-dosed anticholinergic. When applied to the axillae, they reduce sweating in patients nine years and older with hyperhidrosis.1
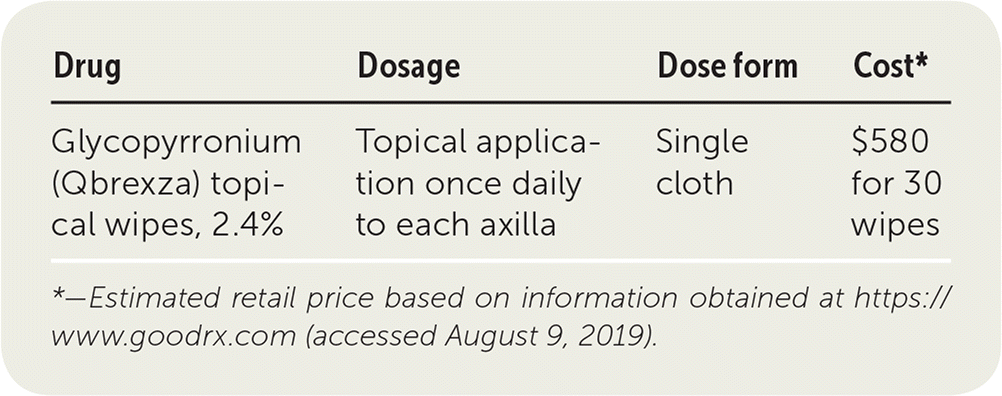
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Glycopyrronium (Qbrexza) topical wipes, 2.4% | Topical application once daily to each axilla | Single cloth | $580 for 30 wipes |
Safety
Systemic anticholinergic absorption may occur with use of glycopyrronium wipes. The most serious adverse effects of glycopyrronium wipes are blurred vision and dehydration. In a study of more than 450 patients, nearly one in 25 patients experienced blurred vision. Dehydration occurred in only one patient.1 There is a theoretic risk of worsening narrow-angle glaucoma, paralytic ileus, urinary retention, and myasthenia gravis, as well as an increased risk of heat injury.2 Coadministration of glycopyrronium wipes and other anticholinergic medications should be avoided. Safety of glycopyrronium wipes has not been studied in children younger than nine years, women who are pregnant or breastfeeding, or patients with significant renal impairment.
Tolerability
Minor adverse effects are common. In two clinical trials of 459 patients treated with glycopyrronium over four weeks, more than one-third experienced minor adverse effects.1 The most common is dry mouth, reported by one in four patients.1 Dilated pupils are less common (7%) but can be unilateral, falsely raising concern for neurologic injury.1,2 With long-term use of glycopyrronium, adverse effects will be reported by 59% of patients, leading to 8% of patients discontinuing treatment.3
Effectiveness
Glycopyrronium wipes decrease symptoms of hyperhidrosis more effectively than control wipes, with one additional person benefiting after four weeks of use compared with placebo (number needed to treat = 3).1 Improvement, defined as a change of four points on a 10-point sweating scale, will occur in 60% of patients using glycopyrronium wipes, compared with approximately 25% of patients using control wipes.1 Although not directly compared with glycopyrronium, aluminum chloride is effective in up to 87% of patients with hyperhidrosis, and onabotulinumtoxinA (Botox) injections have a 94% success rate at four weeks.4 Glycopyrronium treatment continues to be effective for at least 44 weeks with regular use. Glycopyrronium wipes have been studied and labeled for the primary treatment of hyperhidrosis of the axillae, but no other sites.2
Price
A one-month supply of 30 glycopyrronium wipes (one box) costs approximately $580. This is significantly more expensive than topical aluminum chloride hexahydrate therapies such as Drysol, which costs about $15 for one 35-mL bottle. The price of glycopyrronium also exceeds the price of a 50-unit vial of onabotulinumtoxinA, which is given via injection every four months and costs about $350. Glycopyrronium wipes are on the Medicare part D formulary, but they may not be covered by all insurance plans.
Simplicity
Patients should be instructed to apply glycopyrronium to each axilla using a single cloth once daily. The instructions specify a single pass on each axilla. Neither dosage nor timing should be adjusted. Following use, patients should wash their hands immediately with soap and water to remove the medication and reduce the risk of adverse effects such as blurred vision.5
Bottom Line
Glycopyrronium wipes are a useful alternative for the topical treatment of hyperhidrosis of the axillae in patients nine years and older who do not respond to or tolerate nonprescription antiperspirants or topical aluminum chloride. However, given their high cost and potential for adverse effects, glycopyrronium wipes should not be prescribed as a first choice.
The views expressed are those of the authors and do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. government.