Am Fam Physician. 2019;100(6):378-379
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• A 10- to 21-day course of dual antiplatelet therapy reduces stroke recurrence and improves quality of life after mild stroke or high-risk TIA.
• Low-dose aspirin and a 300-mg loading dose of clopidogrel should be started as soon as imaging rules out hemorrhage.
• After 10 to 21 days of daily low-dose aspirin and clopidogrel, 75 mg, the patient should be switched to a single antiplatelet drug.
From the AFP Editors
Dual antiplatelet therapy after stroke has not previously been shown to improve outcomes over a single agent. Based on a recent randomized controlled trial followed by a systematic review, the BMJ and MAGIC group concluded that dual antiplatelet therapy use for a limited period after mild stroke is beneficial. The combination of low-dose aspirin and clopidogrel (Plavix) reduces recurrent stroke and disability compared with aspirin alone when started as soon as possible after a high-risk transient ischemic attack (TIA) or minor ischemic stroke without persistent disabling neurologic deficit and continued for 10 to 21 days.
The severity of TIA can be determined using the ABCD2 score (Table 1). Dual antiplatelet therapy is recommended for an ABCD2 score of 4 or greater. Minor stroke can be identified by a National Institutes of Health (NIH) Stroke Scale score of 3 or less. The risk of recurrence after minor stroke is similar to that after a high-risk TIA. The NIH Stroke Scale ranges from 0 to 42 and is based on measures of motor and sensory function, language and speech, vision, level of consciousness and attention, and neglect. Dual antiplatelet therapy should be started as soon as brain imaging rules out intracranial hemorrhage. Although trials used various dosing strategies, members of the BMJ and MAGIC panel recommend a loading dose of 300 mg of clopidogrel followed by 75 mg daily, and low-dose aspirin at 75 to 81 mg daily. The aspirin should be taken whole without food, but clopidogrel can be crushed or split and taken with or without food.
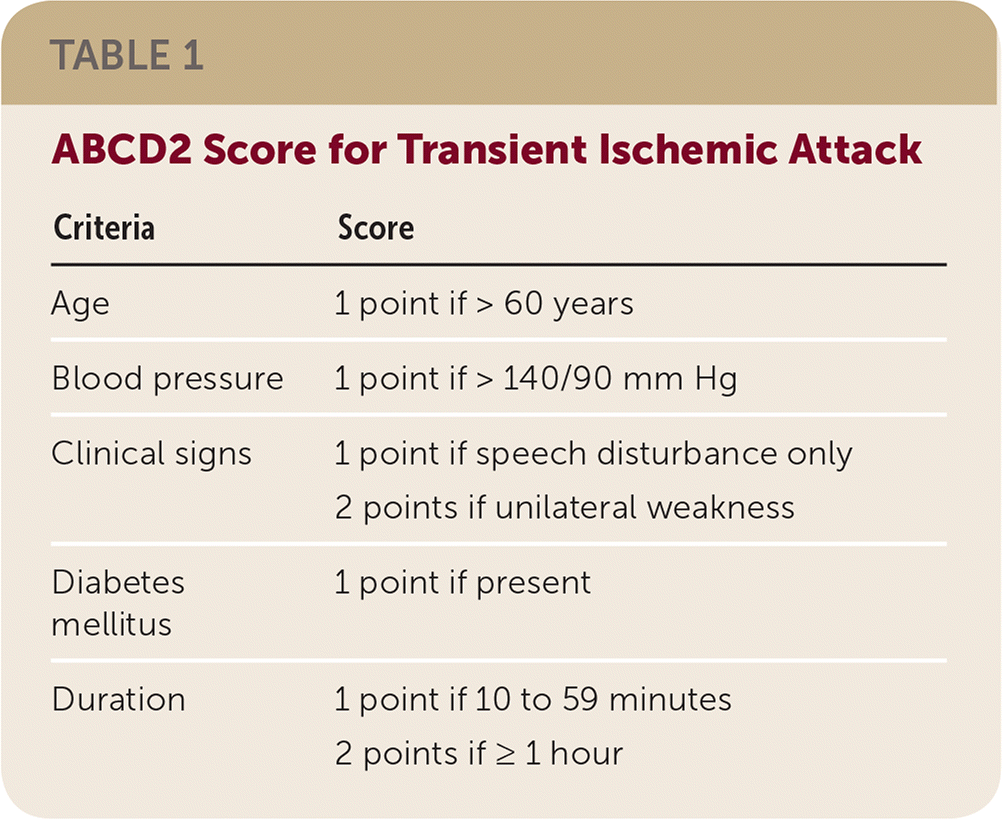
| Criteria | Score |
|---|---|
| Age | 1 point if > 60 years |
| Blood pressure | 1 point if > 140/90 mm Hg |
| Clinical signs | 1 point if speech disturbance only |
| 2 points if unilateral weakness | |
| Diabetes mellitus | 1 point if present |
| Duration | 1 point if 10 to 59 minutes |
| 2 points if ≥ 1 hour |
When imaging will be performed more than 24 hours after symptom onset, treatment should be initiated as soon as minor ischemic stroke or transient TIA is diagnosed by a physician with intent to image as soon as possible.
Evidence indicates that there is no improvement in stroke-related outcomes and increased risk of bleeding with continuation of dual anti-platelet therapy in the long term (22 to 90 days) after stroke. Patients, however, should likely continue to take one agent for the forseeable future. Dual antiplatelet therapy should not be used in patients experiencing a major stroke because of the associated increased risk of intracranial bleeding.
Background
The systematic review on which this recommendation was based included three randomized controlled trials evaluating dual antiplatelet therapy compared with aspirin monotherapy in more than 10,000 patients. These studies identified that dual therapy decreased nonfatal recurrent strokes (number needed to treat [NNT] = 53), moderate to severe disability (NNT = 72), and poor quality of life (NNT = 77). Dual antiplatelet therapy had no effect on all-cause mortality or the incidence of myocardial infarction or recurrent TIA, and had some associated harms of minor (number needed to harm [NNH] = 143) and moderate to major (NNH = 500) extracranial bleeding.
The panel believed that most patients would value preventing another stroke over experiencing bleeding and thus opt for dual therapy over monotherapy. They also believed most patients would opt for shorter treatment duration because of the similar benefits provided, with less associated harm.
Editor's Note: The numbers needed to treat and to harm were calculated by the AFP medical editors based on raw data provided in the original BMJ article.
Guideline source: The BMJ and MAGIC Group
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Yes
Recommendations based on patient-oriented outcomes? Yes
Published source: BMJ. December 2018;363:k5130
Available at: https://www.bmj.com/content/363/bmj.k5130.long