
Am Fam Physician. 2019;100(10):online
Related USPSTF: Preexposure Prophylaxis for the Prevention of HIV Infection: Recommendation Statement
Related Putting Prevention into Practice: Screening for HIV Infection and Preexposure Prophylaxis for the Prevention of HIV Infection
Summary of Recommendation and Evidence
The USPSTF recommends that clinicians screen for HIV infection in adolescents and adults aged 15 to 65 years. Younger adolescents and older adults who are at increased risk of infection should also be screened (Table 1). A recommendation.
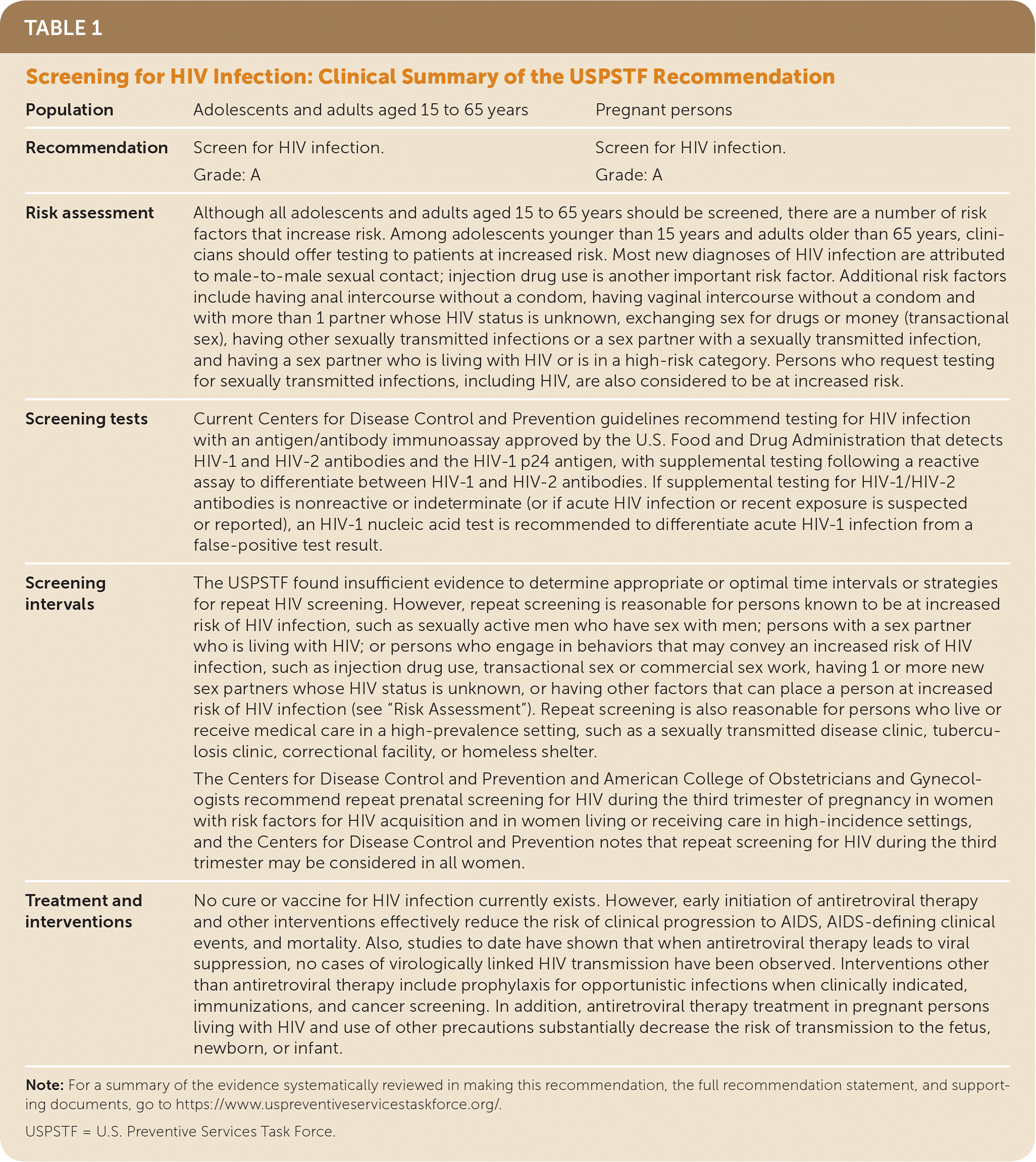
| Population | Adolescents and adults aged 15 to 65 years | Pregnant persons |
| Recommendation | Screen for HIV infection. Grade: A | Screen for HIV infection. Grade: A |
| Risk assessment | Although all adolescents and adults aged 15 to 65 years should be screened, there are a number of risk factors that increase risk. Among adolescents younger than 15 years and adults older than 65 years, clinicians should offer testing to patients at increased risk. Most new diagnoses of HIV infection are attributed to male-to-male sexual contact; injection drug use is another important risk factor. Additional risk factors include having anal intercourse without a condom, having vaginal intercourse without a condom and with more than 1 partner whose HIV status is unknown, exchanging sex for drugs or money (transactional sex), having other sexually transmitted infections or a sex partner with a sexually transmitted infection, and having a sex partner who is living with HIV or is in a high-risk category. Persons who request testing for sexually transmitted infections, including HIV, are also considered to be at increased risk. | |
| Screening tests | Current Centers for Disease Control and Prevention guidelines recommend testing for HIV infection with an antigen/antibody immunoassay approved by the U.S. Food and Drug Administration that detects HIV-1 and HIV-2 antibodies and the HIV-1 p24 antigen, with supplemental testing following a reactive assay to differentiate between HIV-1 and HIV-2 antibodies. If supplemental testing for HIV-1/HIV-2 antibodies is nonreactive or indeterminate (or if acute HIV infection or recent exposure is suspected or reported), an HIV-1 nucleic acid test is recommended to differentiate acute HIV-1 infection from a false-positive test result. | |
| Screening intervals | The USPSTF found insufficient evidence to determine appropriate or optimal time intervals or strategies for repeat HIV screening. However, repeat screening is reasonable for persons known to be at increased risk of HIV infection, such as sexually active men who have sex with men; persons with a sex partner who is living with HIV; or persons who engage in behaviors that may convey an increased risk of HIV infection, such as injection drug use, transactional sex or commercial sex work, having 1 or more new sex partners whose HIV status is unknown, or having other factors that can place a person at increased risk of HIV infection (see “Risk Assessment”). Repeat screening is also reasonable for persons who live or receive medical care in a high-prevalence setting, such as a sexually transmitted disease clinic, tuberculosis clinic, correctional facility, or homeless shelter. The Centers for Disease Control and Prevention and American College of Obstetricians and Gynecologists recommend repeat prenatal screening for HIV during the third trimester of pregnancy in women with risk factors for HIV acquisition and in women living or receiving care in high-incidence settings, and the Centers for Disease Control and Prevention notes that repeat screening for HIV during the third trimester may be considered in all women. | |
| Treatment andinterventions | No cure or vaccine for HIV infection currently exists. However, early initiation of antiretroviral therapy and other interventions effectively reduce the risk of clinical progression to AIDS, AIDS-defining clinical events, and mortality. Also, studies to date have shown that when antiretroviral therapy leads to viral suppression, no cases of virologically linked HIV transmission have been observed. Interventions other than antiretroviral therapy include prophylaxis for opportunistic infections when clinically indicated, immunizations, and cancer screening. In addition, antiretroviral therapy treatment in pregnant persons living with HIV and use of other precautions substantially decrease the risk of transmission to the fetus, newborn, or infant. | |
The USPSTF recommends that clinicians screen for HIV infection in all pregnant persons, including those who present in labor or at delivery whose HIV status is unknown. A recommendation.
See the Clinical Considerations section for more information about assessment of risk, screening intervals, and rescreening in pregnancy.
Rationale
IMPORTANCE
Approximately 1.1 million persons in the United States are currently living with HIV,1 and more than 700,000 persons have died of AIDS since the first cases were reported in 1981.2 The estimated prevalence of HIV infection among persons 13 years and older in the United States is 0.4% (0.7% in males and 0.2% in females),3 and data from the Centers for Disease Control and Prevention (CDC) 2017 HIV Surveillance Report show a significant increase in HIV diagnoses starting at age 15 years (compared with ages 13–14 years).2 The annual number of new cases of HIV infection diagnosed in the United States has decreased slightly in recent years, from about 41,200 new cases in 2012 to 38,300 in 2017.2 Approximately 15% of persons living with HIV are unaware of their infection.1,3,4 It is estimated that persons unaware of their HIV status are responsible for 40% of transmission of HIV in the United States.4
An estimated 8,700 women living with HIV give birth each year in the United States.5 HIV can be transmitted from mother to child during pregnancy, labor, delivery, and breastfeeding. The incidence of perinatal HIV infection in the United States peaked in 19926 and has declined significantly following the implementation of routine prenatal HIV screening and the use of effective therapies and precautions to prevent mother-to-child transmission. Nearly 22,000 perinatal infections were prevented between 1994 and 2010 because of screening and preventive measures.7
DETECTION
The USPSTF found convincing evidence that currently recommended HIV tests are highly accurate in diagnosing HIV infection.
BENEFITS OF DETECTION AND EARLY TREATMENT
The USPSTF found convincing evidence that identification and early treatment of HIV infection is of substantial benefit in reducing the risk of AIDS-related events or death. The USPSTF found convincing evidence that the use of antiretroviral therapy (ART) is of substantial benefit in decreasing the risk of HIV transmission to uninfected sex partners. The USPSTF also found convincing evidence that identification and treatment of pregnant women living with HIV infection are of substantial benefit in reducing the rate of mother-to-child transmission. The overall magnitude of the benefit of screening for HIV infection in adolescents, adults, and pregnant women is substantial.
HARMS OF DETECTION AND EARLY TREATMENT
The USPSTF found adequate evidence that individual antiretroviral drugs, ART drug classes, and ART combinations are associated with some harms, including neuropsychiatric, renal, and hepatic harms and an increased risk of preterm birth in pregnant women. The overall magnitude of the harms of screening for and treatment of screen-detected HIV infection in adolescents, adults, and pregnant women is small.
USPSTF ASSESSMENT
The USPSTF concludes with high certainty that the net benefit of screening for HIV infection in adolescents, adults, and pregnant women is substantial.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to adolescents, adults, and all pregnant persons regardless of age. Based on the age-stratified incidence of HIV infection and data on sexual activity in youth, the USPSTF recommends screening for HIV infection beginning at age 15 years. Adolescents younger than 15 years and adults older than 65 years should be screened if they have risk factors for HIV infection.
ASSESSMENT OF RISK
Although all adolescents and adults aged 15 to 65 years should be screened, there are a number of risk factors that increase risk. Among adolescents younger than 15 years and adults older than 65 years, clinicians should consider the risk factors of their patients, especially those with new sex partners, and offer testing to patients at increased risk.
Most (67%) new diagnoses of HIV infection are attributed to male-to-male sexual contact,2 and the estimated prevalence of HIV infection among men who have sex with men is 12%.3 Injection drug use is another important risk factor for HIV infection; the estimated prevalence of HIV infection among persons who inject drugs is 1.9%.3 In 2017, male individuals 13 years and older accounted for 81% of new diagnoses of HIV infection.2 Most (83%) of these new diagnoses of HIV infection were attributed to male-to-male sexual contact, while 9% were attributed to heterosexual contact, 4% to injection drug use, and 4% to both male-to-male sexual contact and injection drug use.2 Among female individuals 13 years and older, 87% of all new diagnoses were attributed to heterosexual contact and 12% to injection drug use.2
Additional risk factors for HIV infection include having anal intercourse without a condom, having vaginal intercourse without a condom and with more than 1 partner whose HIV status is unknown, exchanging sex for drugs or money (transactional sex), having other sexually transmitted infections (STIs) or a sex partner with an STI, and having a sex partner who is living with HIV or is in a high-risk category. Persons who request testing for STIs, including HIV, are also considered at increased risk.
The USPSTF recognizes that these risk categories are not mutually exclusive, that the degree of risk exists on a continuum, and that persons may not be aware of the HIV or risk status of their sex partner or the person with whom they share injection drug equipment. Patients may also be reluctant to disclose risk factors to clinicians.
SCREENING TESTS
Current CDC guidelines recommend testing for HIV infection with an antigen/antibody immunoassay approved by the U.S. Food and Drug Administration that detects HIV-1 and HIV-2 antibodies and the HIV-1 p24 antigen, with supplemental testing after a reactive assay to differentiate between HIV-1 and HIV-2 antibodies.8,9 If supplemental testing for HIV-1/HIV-2 antibodies is nonreactive or indeterminate (or if acute HIV infection or recent exposure is suspected or reported), an HIV-1 nucleic acid test is recommended to differentiate acute HIV-1 infection from a false-positive test result.8,9
When using a rapid HIV test for screening, positive results should be confirmed. Pregnant women presenting in labor with unknown HIV status should be screened with a rapid HIV test to get results as soon as possible.
SCREENING INTERVALS
The USPSTF found insufficient evidence to determine appropriate or optimal time intervals or strategies for repeat HIV screening. Repeat screening is reasonable for persons known to be at increased risk of HIV infection, such as sexually active men who have sex with men; persons with a sex partner who is living with HIV; or persons who engage in behaviors that may convey an increased risk of HIV infection, such as injection drug use, transactional sex or commercial sex work, having 1 or more new (i.e., since a prior HIV test) sex partners whose HIV status is unknown, or having other factors that can place a person at increased risk of HIV infection (see the Assessment of Risk section). Repeat screening is also reasonable for persons who live or receive medical care in a high-prevalence setting, such as a sexually transmitted disease clinic, tuberculosis clinic, correctional facility, or homeless shelter. The CDC recommends annual screening in persons at increased risk10 but recognizes that clinicians may wish to screen high-risk men who have sex with men more frequently (e.g., every 3 or 6 months) depending on the patient's risk factors, local HIV prevalence, and local policies.11 Routine rescreening may not be necessary for persons who have not been at increased risk since they last tested negative for HIV.
The USPSTF found no evidence on the yield of repeat prenatal screening for HIV compared with 1-time screening during a single pregnancy. The CDC10 and the American College of Obstetricians and Gynecologists12 recommend repeat prenatal screening for HIV during the third trimester of pregnancy in women with risk factors for HIV acquisition and in women living or receiving care in high-incidence settings, and the CDC notes that repeat screening for HIV during the third trimester in all women who test negative early in pregnancy may be considered. Women screened during a previous pregnancy should be rescreened in subsequent pregnancies.
TREATMENT
No cure or vaccine for HIV infection currently exists. However, early initiation of ART and other interventions effectively reduce the risk of clinical progression to AIDS, AIDS-defining clinical events, and mortality. Also, studies to date have shown that when ART leads to viral suppression, no cases of virologically linked HIV transmission have been observed. Interventions other than ART include prophylaxis for opportunistic infections when clinically indicated, immunizations, and cancer screening. In addition, ART treatment in pregnant women living with HIV and use of other precautions substantially decrease the risk of transmission to the fetus, newborn, or infant.
The clinical treatment of HIV infection is a dynamic scientific field. The Panel on Antiretroviral Guidelines for Adults and Adolescents of the U.S. Department of Health and Human Services regularly updates guidelines for HIV treatment regimens.13
ADDITIONAL APPROACHES TO PREVENTION
The USPSTF recognizes that the most effective strategy for reducing HIV-related morbidity and mortality in the United States is primary prevention or avoidance of exposure to HIV infection. Avoiding behaviors that may convey an increased risk of HIV infection and consistent use of condoms can decrease the risk of transmission of HIV and other STIs. The USPSTF recommends providing intensive behavioral counseling for all sexually active adolescents and for adults at increased risk of STIs.14
The Community Preventive Ser vices Task Force has made several recommendations related to the prevention of HIV/AIDS and other STIs.15
Prophylactic intervention with antiretroviral medications, both preexposure and postexposure, can prevent HIV infection. Postexposure prophylaxis is used in persons who do not have HIV and may have been exposed to it via sexual contact, occupational or nonoccupational needle stick or other injury, or sharing injection drug equipment. When initiated soon after possible exposure, postexposure prophylaxis can prevent HIV infection. Preexposure prophylaxis is used in persons who do not have HIV and are at high risk of acquiring HIV infection. It consists of antiretroviral medication taken every day, before potential exposure. The USPSTF recommends offering preexposure prophylaxis to persons at high risk of HIV acquisition.16
USEFUL RESOURCES
More information about HIV and AIDS is available at HIV.gov17 and from the CDC.18 The CDC has made recommendations on screening for HIV in adolescents, adults, and pregnant women in health care settings10 and the prevention of HIV transmission in adolescents and adults living with HIV19; guidelines on the use of ART and the potential adverse effects of ART are regularly updated at https://www.aidsinfo.nih.gov.13
