Am Fam Physician. 2019;100(11):online
Author disclosure: No relevant financial affiliations.

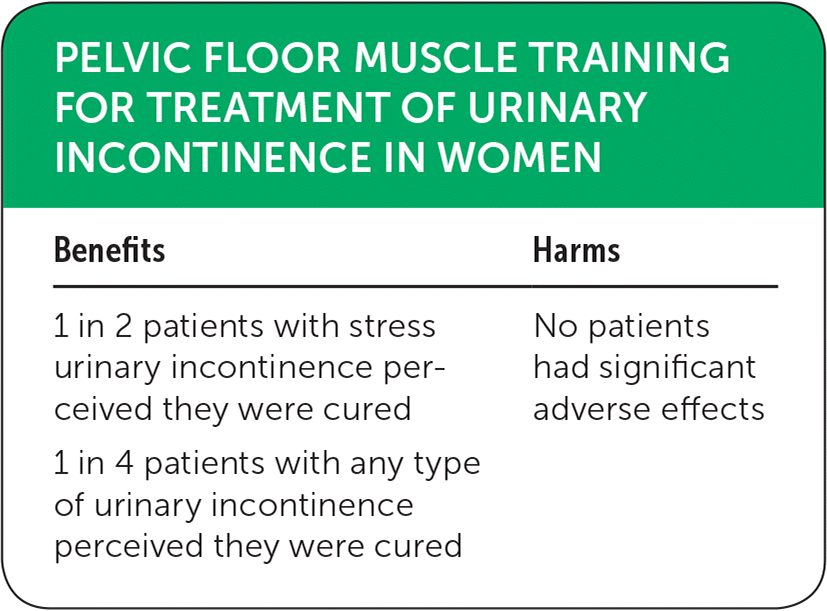
| Benefits | Harms |
|---|---|
| 1 in 2 patients with stress urinary incontinence perceived they were cured | No patients had significant adverse effects |
| 1 in 4 patients with any type of urinary incontinence perceived they were cured |
Details for This Review
Study Population: Adult women with stress urinary incontinence (UI), urge UI, or mixed UI
Efficacy End Points: Patient-perceived cure or improvement, symptoms, quality of life, frequency of leakage episodes, amount of urine lost
Harm End Points: Discomfort, soreness, pain, bleeding
Narrative: UI is common in adult women, leading to health complications such as rash and urinary tract infections. UI can also lead to a decrease in social and physical activities as well as impaired emotional and psychological well-being. There are two main causes of UI. The first cause is neuromuscular, involving pelvic floor weakness, which results in suboptimal positioning of the urethra and inability to close the bladder outlet (stress UI). The second cause is overactivity of the detrusor muscle, which leads to increased pressure and inability to close the bladder outlet accompanied by the immediate urge to void (urge UI). Pelvic floor muscle training (PFMT) seeks to reverse this weakness and facilitate timely contraction of these muscles. There are multiple types of PFMT programs, with or without a biofeedback component. PFMT is considered first-line therapy for all types of urinary incontinence due to the significant adverse effects of the anticholinergic medications that are commonly prescribed to treat UI.
A 2018 Cochrane review of 31 trials (1,817 participants) evaluated the effectiveness of PFMT to treat UI vs. no treatment.1 The study population included women with stress UI, urge UI, and mixed UI. Most of the patients had stress UI, so the analyses were conducted on patients with stress UI and all types of UI. There was little additional information about the demographics of the study population. The studies assessed outcomes of patient-perceived cure, patient-perceived improvement after treatment, number of leakage episodes in 24 hours (assessed by a journal), or short (up to one hour) pad test measured in grams of urine. Treatment duration ranged from six weeks to six months.
This meta-analysis found significant improvement in all outcomes. Patients with stress UI perceived cure 56% of the time with PFMT compared with 6% of women in the comparison group (number needed to treat [NNT] = 2; 95% CI, 1.06 to 6.17). Cure was defined as the greater degree of improvement of symptoms or no symptoms (e.g., staying dry all day) and was reported using multiple definitions describing the degree of impairment. This was the only outcome for which the evidence was considered high quality. Patients with stress UI perceived cure or improvement 72% of the time in the PFMT group compared with 11.4% in the comparison group (NNT = 2; 95% CI, 1.13 to 3.05) Patients with all types of UI in the PFMT group perceived cure 32.9% of the time compared with 6.2% of women in the comparison group (NNT = 4; 95% CI, 1.75 to 9.17). Grams of urine lost during a short (up to one hour) pad test decreased by 9.71 g (95% CI, 0.5 to 18.92) for women with stress UI. The remaining outcomes were rated as moderate quality. Seven trials reported adverse effects, which were classified as mild and were not increased in the PFMT group. PFMT for treatment of UI is rated as green despite the poor-quality studies because of the large effect size and minimal adverse effects.
Caveats: In general, the studies included were low quality and included small numbers of patients. Interventions and outcomes measured were heterogeneous. Only one of the conclusions (patient-perceived cure of stress UI) was based on high-quality evidence. Blinding was difficult in these studies because of the nature of the intervention. The included studies generally compared PFMT with no treatment rather than a sham treatment or placebo. This limitation introduced the possibility of a placebo effect.