Am Fam Physician. 2020;101(2):121-122
Author disclosure: No relevant financial affiliations.
Revefenacin (Yupelri) is a once-daily, nebulized, long-acting muscarinic antagonist labeled for the treatment of moderate to severe chronic obstructive pulmonary disease (COPD).
Safety
Revefenacin is generally safe with minimal systemic adverse effects.1 As with other long-acting treatments, revefenacin should not be used to treat acute symptoms. Similar to other inhaled therapies, it has a theoretic risk of immediate hypersensitivity reaction and acute bronchospasm. Revefenacin is anticholinergic and may induce narrow-angle glaucoma or worsen urinary retention. Revefenacin has not been studied in pregnant women or breastfed infants. The manufacturer recommends against its use in patients with hepatic impairment or those taking rifampin or cyclosporine (Sandimmune).
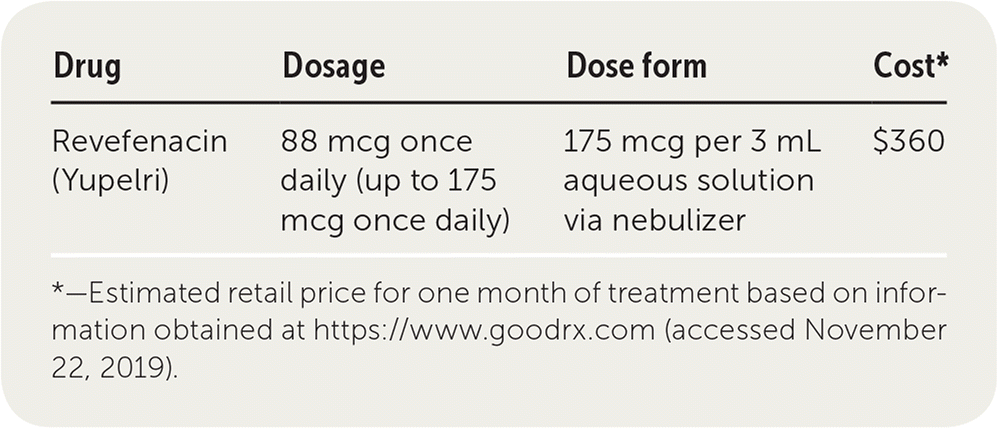
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Revefenacin (Yupelri) | 175 mcg once daily | 175 mcg per 3 mL aqueous solution via nebulizer | $1,000 |
Tolerability
Revefenacin was shown to be well tolerated in clinical trials. In two 12-week placebo-controlled trials of 1,225 patients with moderate to severe COPD, adverse effects leading to discontinuation were more common in patients receiving placebo (19%) than revefenacin (13%).2
Effectiveness
Revefenacin is superior to placebo in improving forced expiratory volume in one second (FEV1) for patients with moderate to severe COPD. A 28-day randomized, double-blind study compared placebo and revefenacin in 355 patients with COPD, a mean age of 62 years, and a mean FEV1 of 44% of predicted. Patients were allowed to use inhaled corticosteroids and short-acting bronchodilators.3 Revefenacin improved FEV1 by at least 100 mL from baseline with effects lasting four hours or more; participants' mean number of albuterol puffs also decreased from three to two per day when using revefenacin. These results were replicated in an 85-day randomized, double-blind study (619 patients older than 40 years) in which participants were also allowed to use long-acting muscarinic antagonists and inhaled steroid therapy.4 This study demonstrated improvements in FEV1 that were sustained over the entire study period, but revefenacin has not been evaluated for its effect in decreasing exacerbations, hospitalization rates, mortality, functional status, and quality of life.
Price
Revefenacin costs approximately $1,000 per month, compared with about $415 per month for common inhaled long-acting muscarinic antagonists such as tiotropium (Spiriva). This cost is more than that of most commonly used COPD medications. Revefenacin may require prior authorization or formulary exemption to obtain insurance coverage.
Simplicity
Revefenacin is administered as a nebulized solution once daily over eight minutes. The daily dosage is 175 mcg. Patients should be instructed on how to use the accompanying nebulizer. The sterile solution is provided in individual vials.
Bottom Line
Revefenacin is a once-daily nebulized alternative to metered dose long-acting muscarinic antagonists for patients with moderate to severe COPD. Given its expense, complicated administration process, and lack of evidence that it improves key patient-oriented outcomes, its use should be limited to patients who are unable to use a metered dose inhaler.