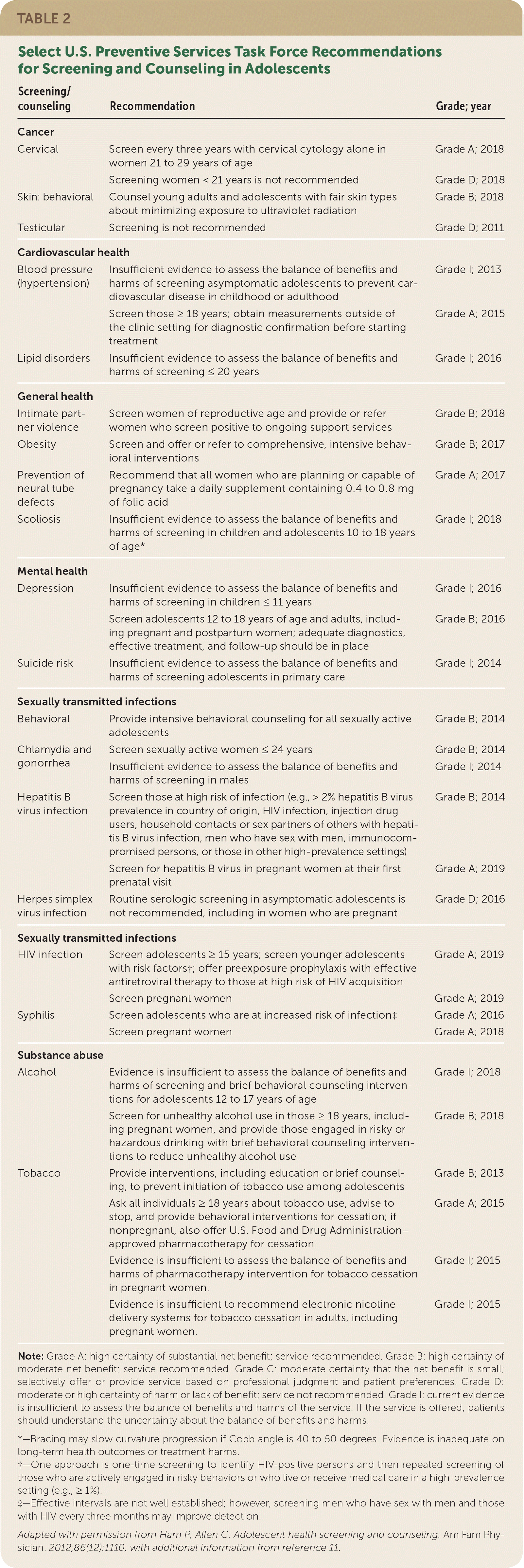
| Cancer |
| Cervical | Screen every three years with cervical cytology alone in women 21 to 29 years of age | Grade A; 2018 |
| Screening women < 21 years is not recommended | Grade D; 2018 |
| Skin: behavioral | Counsel young adults and adolescents with fair skin types about minimizing exposure to ultraviolet radiation | Grade B; 2018 |
| Testicular | Screening is not recommended | Grade D; 2011 |
| Cardiovascular health |
| Blood pressure (hypertension) | Insufficient evidence to assess the balance of benefits and harms of screening asymptomatic adolescents to prevent cardiovascular disease in childhood or adulthood | Grade I; 2013 |
| Screen those ≥ 18 years; obtain measurements outside of the clinic setting for diagnostic confirmation before starting treatment | Grade A; 2015 |
| Lipid disorders | Insufficient evidence to assess the balance of benefits and harms of screening ≤ 20 years | Grade I; 2016 |
| General health |
| Intimate partner violence | Screen women of reproductive age and provide or refer women who screen positive to ongoing support services | Grade B; 2018 |
| Obesity | Screen and offer or refer to comprehensive, intensive behavioral interventions | Grade B; 2017 |
| Prevention of neural tube defects | Recommend that all women who are planning or capable of pregnancy take a daily supplement containing 0.4 to 0.8 mg of folic acid | Grade A; 2017 |
| Scoliosis | Insufficient evidence to assess the balance of benefits and harms of screening in children and adolescents 10 to 18 years of age* | Grade I; 2018 |
| Mental health |
| Depression | Insufficient evidence to assess the balance of benefits and harms of screening in children ≤ 11 years | Grade I; 2016 |
| Screen adolescents 12 to 18 years of age and adults, including pregnant and postpartum women; adequate diagnostics, effective treatment, and follow-up should be in place | Grade B; 2016 |
| Suicide risk | Insufficient evidence to assess the balance of benefits and harms of screening adolescents in primary care | Grade I; 2014 |
| Sexually transmitted infections |
| Behavioral | Provide intensive behavioral counseling for all sexually active adolescents | Grade B; 2014 |
| Chlamydia and gonorrhea | Screen sexually active women ≤ 24 years | Grade B; 2014 |
| Insufficient evidence to assess the balance of benefits and harms of screening in males | Grade I; 2014 |
| Hepatitis B virus infection | Screen those at high risk of infection (e.g., > 2% hepatitis B virus prevalence in country of origin, HIV infection, injection drug users, household contacts or sex partners of others with hepatitis B virus infection, men who have sex with men, immunocompromised persons, or those in other high-prevalence settings) | Grade B; 2014 |
| Screen for hepatitis B virus in pregnant women at their first prenatal visit | Grade A; 2019 |
| Herpes simplex virus infection | Routine serologic screening in asymptomatic adolescents is not recommended, including in women who are pregnant | Grade D; 2016 |
| HIV infection | Screen adolescents ≥ 15 years; screen younger adolescents with risk factors†; offer preexposure prophylaxis with effective antiretroviral therapy to those at high risk of HIV acquisition | Grade A; 2019 |
| Screen pregnant women | Grade A; 2019 |
| Syphilis | Screen adolescents who are at increased risk of infection‡ | Grade A; 2016 |
| Screen pregnant women | Grade A; 2018 |
| Substance abuse |
| Alcohol | Evidence is insufficient to assess the balance of benefits and harms of screening and brief behavioral counseling interventions for adolescents 12 to 17 years of age | Grade I; 2018 |
| Screen for unhealthy alcohol use in those ≥ 18 years, including pregnant women, and provide those engaged in risky or hazardous drinking with brief behavioral counseling interventions to reduce unhealthy alcohol use | Grade B; 2018 |
| Tobacco | Provide interventions, including education or brief counseling, to prevent initiation of tobacco use among adolescents | Grade B; 2013 |
| Ask all individuals ≥ 18 years about tobacco use, advise to stop, and provide behavioral interventions for cessation; if nonpregnant, also offer U.S. Food and Drug Administration–approved pharmacotherapy for cessation | Grade A; 2015 |
| Evidence is insufficient to assess the balance of benefits and harms of pharmacotherapy intervention for tobacco cessation in pregnant women. | Grade I; 2015 |
| Evidence is insufficient to recommend electronic nicotine delivery systems for tobacco cessation in adults, including pregnant women. | Grade I; 2015 |