Am Fam Physician. 2020;101(4):213-220
Author disclosure: No relevant financial affiliations.
Cerebral palsy, which occurs in two to three out of 1,000 live births, has multiple etiologies resulting in brain injury that affects movement, posture, and balance. The movement disorders associated with cerebral palsy are categorized as spasticity, dyskinesia, ataxia, or mixed/other. Spasticity is the most common movement disorder, occurring in 80% of children with cerebral palsy. Movement disorders of cerebral palsy can result in secondary problems, including hip pain or dislocation, balance problems, hand dysfunction, and equinus deformity. Diagnosis of cerebral palsy is primarily clinical, but magnetic resonance imaging can be helpful to confirm brain injury if there is no clear cause for the patient’s symptoms. Once cerebral palsy has been diagnosed, an instrument such as the Gross Motor Function Classification System can be used to evaluate severity and treatment response. Treatments for the movement disorders associated with cerebral palsy include intramuscular onabotulinumtoxinA, systemic and intrathecal muscle relaxants, selective dorsal rhizotomy, and physical and occupational therapies. Patients with cerebral palsy often also experience problems unrelated to movement that need to be managed into adulthood, including cognitive dysfunction, seizures, pressure ulcers, osteoporosis, behavioral or emotional problems, and speech and hearing impairment. (Am Fam Physician. 2020;101(4):213–220. Copyright © 2020 American Academy of Family Physicians.)
The Centers for Disease Control and Prevention defines cerebral palsy as a group of disorders that affects an individual’s movement, posture, and balance.1 The clinical findings, which are due to an injury to the developing brain, are permanent and nonprogressive, but they can change over time.
WHAT’S NEW ON THIS TOPIC
Cerebral Palsy
Although selective dorsal rhizotomy is typically used for ambulatory spastic diplegia in children with Gross Motor Function Classification System level II or III cerebral palsy, more recent data suggest that it may also be helpful for more severe cases.
Assessment using a spasticity-related hip surveillance program combined with early, preventive surgical release has been demonstrated to reduce hip pain, hip dislocation, and the need for orthopedic salvage surgery.
Etiology
Cerebral palsy has multiple etiologies that can affect different parts of the brain, thus contributing to the broad range of clinical findings. Approximately 92% of cases of cerebral palsy are traced to the perinatal period.3 Risk factors include preterm birth, perinatal infection (particularly chorioamnionitis), intrauterine growth restriction, use of preterm antibiotics before rupture of membranes, acidosis or asphyxia, and multiple gestation, any of which can lead to brain injury.4,5 Fewer than 10% of cases are attributable to intrapartum hypoxia.6 Cerebral palsy occurs at an older age in about 8% of patients, often from head injury or infection.3 Despite identification of risk factors, 80% of cases have no clear cause and are considered idiopathic.7
Clinical Features
The clinical features of cerebral palsy are varied and encompass a broad range of abnormalities. They are predominantly disorders of movement but also include a spectrum of abnormalities such as poor balance and sensory deficits.1,10 A number of comorbidities that are not part of the core definition of cerebral palsy also occur, most commonly pain (75%), intellectual disability (50%), inability to walk (33%), hip displacement (33%), inability to speak (25%), epilepsy (25%), incontinence (25%), and behavioral or sleep disorders (20% to 25%).11 These clinical findings occur outside of the expected age-based developmental stages. Other studies have shown additional clinical findings such as hearing loss, blindness, and progression of scoliosis due to muscle spasm.10,11
Diagnosis
The diagnosis of cerebral palsy is clinical, based on identification of the defining features.10 The diagnosis can be further classified based on the nature of the movement disorder: stiff muscles (spasticity), uncontrollable movements (dyskinesia), poor coordination (ataxia), or other/mixed.1,7,8,10 Spasticity is the most common movement disorder, affecting approximately 80% of children with cerebral palsy.1 A video demonstrating spasticity and the other movement disorders of cerebral palsy is available at https://www.youtube.com/watch?v=cOfUGUNxEqU. Spasticity in cerebral palsy can be characterized as diplegia, hemiplegia, or quadriplegia, depending on which limbs are affected.
In the past, the diagnosis of cerebral palsy was usually made between 12 and 24 months of age when there were clinical findings of impaired movement, posture, or balance, and it was evident that the impairment was permanent and nonprogressive. However, now that perinatal ultrasonography and postbirth magnetic resonance imaging (MRI) can identify brain injury, the diagnosis may be made as early as six months of age (corrected for prematurity).7,9
The American Academy of Neurology recommends a stepwise workup to aid in the diagnosis of cerebral palsy.9 The first step is recognition of a permanent, nonprogressive disorder of motor function in a child through a history and physical examination. Next, the clinician should screen for the comorbidities that often accompany cerebral palsy.
If perinatal imaging studies, such as fetal anatomy surveys or newborn transcranial ultrasonography, do not show a cause for the clinical findings, neuroimaging may be obtained. MRI is the recommended imaging modality and is preferable to computed tomography given its higher specificity (approximately 89%) for identifying intracranial abnormalities.9 A broad range of abnormalities may be seen on brain imaging in patients with cerebral palsy, including schizencephaly (clefts in cerebral tissue), hydrocephalus, and periventricular leukomalacia. In one study, only 5% of imaging studies demonstrated findings specific to hypoxic-ischemic injury.12
If imaging results are normal or nondiagnostic, the final step is to consider screening for inborn errors of metabolism and carrier states for genetic disorders that might explain the patient’s symptoms.9 However, there is limited evidence to support such testing.
Clinical Assessment Instruments
After establishing the diagnosis, various instruments can be used to evaluate the severity of cerebral palsy and response to treatment. The most widely used evidence-based tool is the Gross Motor Function Classification System (GMFCS).10,14 Other cerebral palsy assessment tools are available, but studies show no major advantages of one over another.15
The GMFCS (available at https://bit.ly/2KtnrCr) is an age-based tool that evaluates gross motor function in different realms, including mobility, posture, and balance, and classifies the severity of each of those realms into one of five levels. Level I indicates few limitations (e.g., walks without limitations), whereas level V indicates severe limitations (e.g., requires a wheelchair).
After classification with the GMFCS, patients may be monitored as they age to see if treatments result in improved GMFCS levels.14 Additional scoring systems such as the Wong-Baker FACES Pain Rating Scale can also be used to assess response to treatment.
Treatment
GENERAL APPROACH
Treatment of patients with cerebral palsy varies depending on the specific symptoms. However, discussing expectations with families to help them develop realistic goals is important in all cases. Involving a multidisciplinary team (Table 116) to address the various aspects of care is also important for tailoring the treatment plan to the patient’s individual needs.
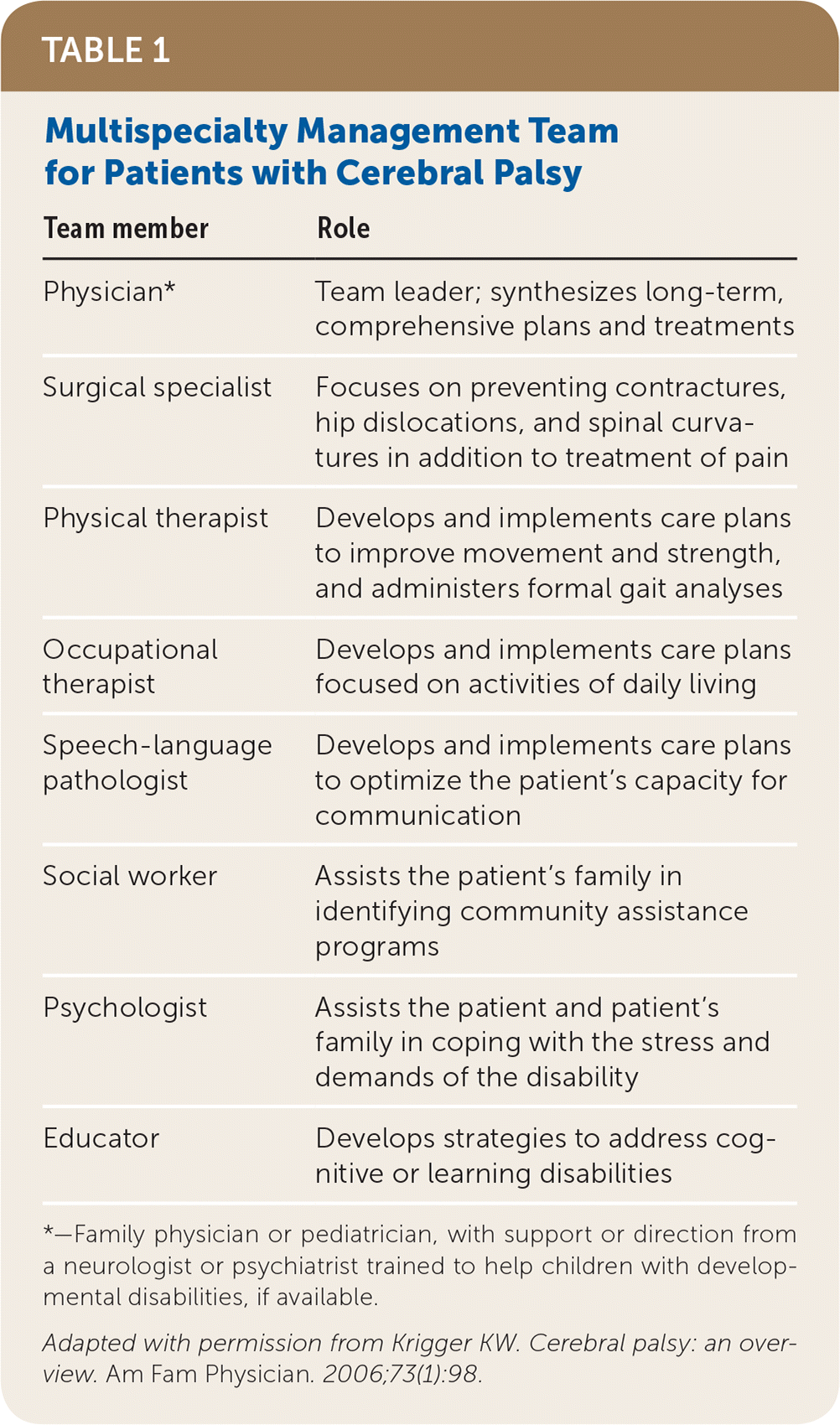
| Team member | Role |
|---|---|
| Physician* | Team leader; synthesizes long-term, comprehensive plans and treatments |
| Surgical specialist | Focuses on preventing contractures, hip dislocations, and spinal curvatures in addition to treatment of pain |
| Physical therapist | Develops and implements care plans to improve movement and strength, and administers formal gait analyses |
| Occupational therapist | Develops and implements care plans focused on activities of daily living |
| Speech-language pathologist | Develops and implements care plans to optimize the patient’s capacity for communication |
| Social worker | Assists the patient’s family in identifying community assistance programs |
| Psychologist | Assists the patient and patient’s family in coping with the stress and demands of the disability |
| Educator | Develops strategies to address cognitive or learning disabilities |
By five years of age, most children with cerebral palsy have about 90% of their eventual total motor development, even with aggressive and ongoing therapy.17 In addition to focusing on motor skills, however, physicians should assist families in coping with development of their child’s communication, social, academic, and eventually professional skills as the child grows into adulthood.17 The treatment of children with cerebral palsy also involves managing the common complications of the condition Table 2.16–23
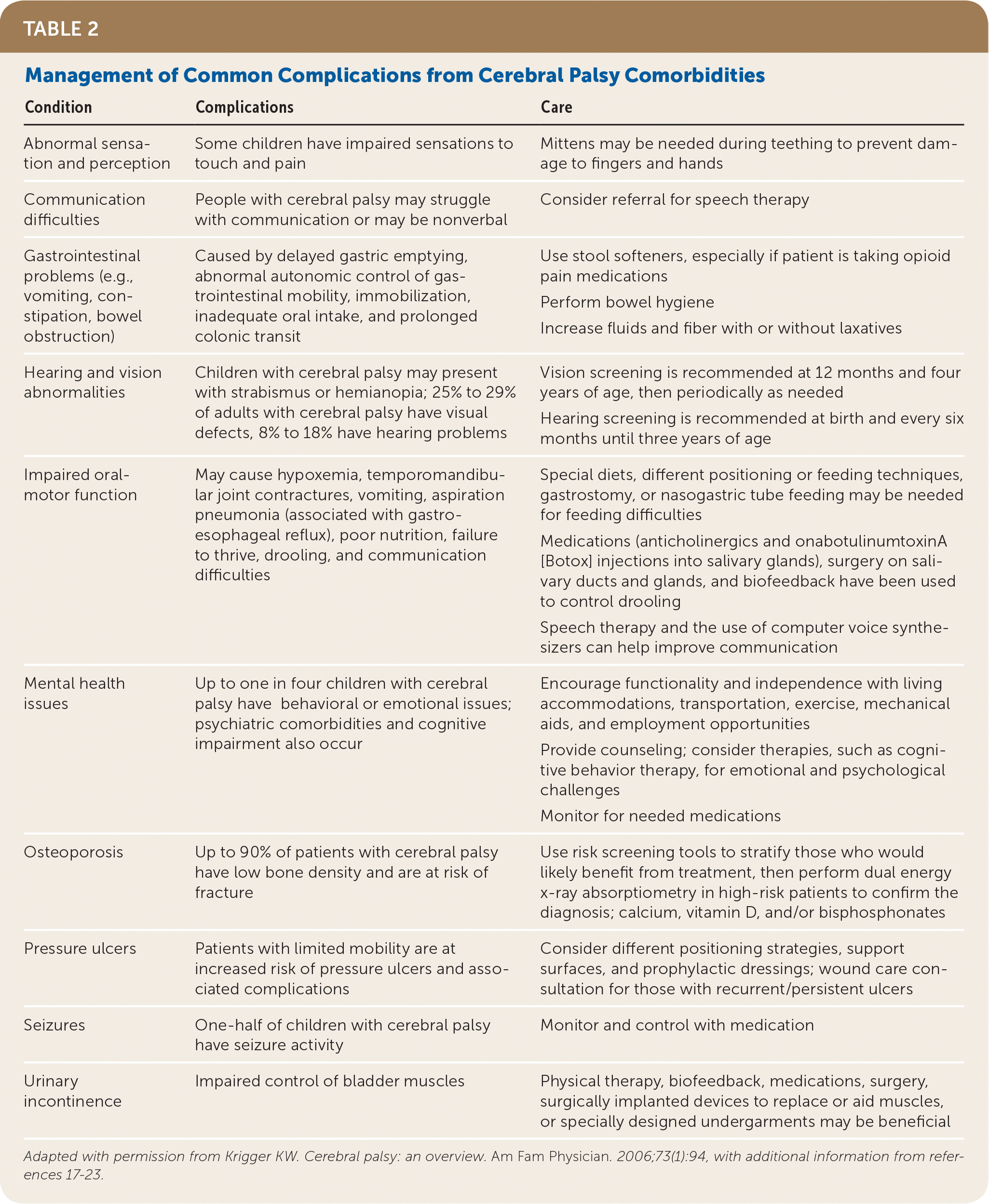
| Condition | Complications | Care |
|---|---|---|
| Abnormal sensation and perception | Some children have impaired sensations to touch and pain | Mittens may be needed during teething to prevent damage to fingers and hands |
| Communication difficulties | People with cerebral palsy may struggle with communication or may be nonverbal | Consider referral for speech therapy |
| Gastrointestinal problems (e.g., vomiting, constipation, bowel obstruction) | Caused by delayed gastric emptying, abnormal autonomic control of gastrointestinal mobility, immobilization, inadequate oral intake, and prolonged colonic transit | Use stool softeners, especially if patient is taking opioid pain medications Perform bowel hygiene Increase fluids and fiber with or without laxatives |
| Hearing and vision abnormalities | Children with cerebral palsy may present with strabismus or hemianopia; 25% to 29% of adults with cerebral palsy have visual defects, 8% to 18% have hearing problems | Vision screening is recommended at 12 months and four years of age, then periodically as needed Hearing screening is recommended at birth and every six months until three years of age |
| Impaired oral-motor function | May cause hypoxemia, temporomandibular joint contractures, vomiting, aspiration pneumonia (associated with gastroesophageal reflux), poor nutrition, failure to thrive, drooling, and communication difficulties | Special diets, different positioning or feeding techniques, gastrostomy, or nasogastric tube feeding may be needed for feeding difficulties Medications (anticholinergics and onabotulinumtoxinA [Botox] injections into salivary glands), surgery on salivary ducts and glands, and biofeedback have been used to control drooling Speech therapy and the use of computer voice synthesizers can help improve communication |
| Mental health issues | Up to one in four children with cerebral palsy have behavioral or emotional issues; psychiatric comorbidities and cognitive impairment also occur | Encourage functionality and independence with living accommodations, transportation, exercise, mechanical aids, and employment opportunities Provide counseling; consider therapies, such as cognitive behavior therapy, for emotional and psychological challenges Monitor for needed medications |
| Osteoporosis | Up to 90% of patients with cerebral palsy have low bone density and are at risk of fracture | Use risk screening tools to stratify those who would likely benefit from treatment, then perform dual energy x-ray absorptiometry in high-risk patients to confirm the diagnosis; calcium, vitamin D, and/or bisphosphonates |
| Pressure ulcers | Patients with limited mobility are at increased risk of pressure ulcers and associated complications | Consider different positioning strategies, support surfaces, and prophylactic dressings; wound care consultation for those with recurrent/persistent ulcers |
| Seizures | One-half of children with cerebral palsy have seizure activity | Monitor and control with medication |
| Urinary incontinence | Impaired control of bladder muscles | Physical therapy, biofeedback, medications, surgery, surgically implanted devices to replace or aid muscles, or specially designed undergarments may be beneficial |
Most treatments for cerebral palsy are supported by weak, short-term evidence. This is largely because of difficulty studying this vulnerable population.
SPASTICITY
Treatment of spasticity is important for preventing and correcting spasticity-induced bone and joint deformation, in addition to controlling pain and maintaining function. Primary care physicians often refer patients to a surgical specialist to aid in selection of appropriate treatments, including nerve blocks, soft tissue lengthening, tendon transfers, and joint stabilization.24 The timing of referral depends on severity. For GMFCS level V cerebral palsy, initial referral should be considered between one and four years of age.25 For GMFCS level I cerebral palsy, initial referral should be considered at around five years of age.25
OnabotulinumtoxinA. Intramuscular onabotulinumtoxinA (Botox) has been used for decades to reduce spasticity and deformity and improve mobility and pain control in children with cerebral palsy of any severity.26 A 2019 Cochrane review indicated mixed outcomes for intramuscular onabotulinumtoxinA with low-quality evidence.27 The optimal age at which to initiate onabotulinumtoxinA injections is controversial, but the first injections typically occur between 18 and 24 months of age.26 European consensus guidelines provide recommendations for the use of onabotulinumtoxinA injections in children with cerebral palsy, including indications, dosing, and techniques.25
Systemic Antispasticity Medications. Medications such as baclofen (Lioresal) and diazepam (Valium) are short-acting drugs that help relax muscle groups, but they come with many adverse effects (e.g., sedation, dizziness, confusion, nausea, lowered seizure threshold, central nervous system depression).28 Although these medications may be necessary in severe cases of cerebral palsy (typically GMFCS level IV or V), there is limited evidence to support their long-term use given the adverse effects.28
Selective Dorsal Rhizotomy. In this neurosurgical procedure, selective nerve roots are severed to reduce spasticity and maximize motor control. Although the procedure is typically used for ambulatory spastic diplegia in children with GMFCS level II or III cerebral palsy, more recent data suggest that it may also be helpful in more severe cases.29 Evaluation for this procedure should be done between four and five years of age.25
Many studies show short-term improvement in gait and range of motion following selective dorsal rhizotomy.30 Long-term studies demonstrate reduced spasticity with the procedure, but functional motor improvement at 10 years is variable.31 Nevertheless, those treated with selective dorsal rhizotomy required significantly less orthopedic surgery and onabotulinumtoxinA injections over 10 or more years of follow-up compared with a matched control group that did not undergo the treatment, along with small improvements in gait outcomes.32
Intrathecal Baclofen. Administration of intrathecal baclofen via an implantable pump is an option that reduces adverse effects by limiting systemic exposure to the drug. It is usually reserved for nonambulatory children with GMFCS level IV or V cerebral palsy. There are few studies to support its use, but it appears to improve quality of life and ease of care in the short term. However, it is expensive, requires refills, is intrusive, and increases risk of infection and surgical complications compared with other treatment options.33
HIP DISORDERS
Hip disorders are among the most common musculoskeletal issues in children with cerebral palsy. Approximately 36% of children with cerebral palsy have a hip disorder, and the incidence increases with higher GMFCS level.34 Spasticity can lead to hip pain and hip dislocation and can make it difficult for families to care for nonambulatory children.
Routine hip surveillance, including periodic examinations and radiography, can help identify developing problems earlier and prevent poor outcomes. The frequency of hip surveillance is determined by GMFCS level. Although no formal hip surveillance program has been developed in the United States, standard-of-care guidelines have been adopted in Europe, Australia, and Canada.35 Assessment using a spasticity-related hip surveillance program combined with early, preventive surgical release has been demonstrated to reduce hip pain, hip dislocation, and the need for orthopedic salvage surgery.34
IMPROVING MOVEMENT AND BALANCE
Formal physical and occupational therapies have been the cornerstones of treatment for movement and balance problems in children and adults with cerebral palsy. There are many different modalities and approaches to therapy, including stretching; massage; strengthening, weight-bearing, and balance exercises; electrical stimulation; treadmill use; and endurance training.
Studies show that physical and occupational therapies improve gait and motor function; however, there is minimal data to support one therapeutic modality over another or to guide the optimal intensity, frequency, or duration of treatment.36,37 Referral for physical and occupational therapies is recommended as soon as cerebral palsy is diagnosed.25 Augmenting therapy with onabotulinumtoxinA injections can further improve motor function in appropriate patients.38
Home therapy programs implemented by parents after a formal instructional session can successfully improve the patient’s function and parent satisfaction.39 Web-based programs that train families on using therapy techniques, with progress monitored by a trained therapist, have also been shown to improve motor skills, although these improvements were limited to the dominant upper limb.40
IMPROVING HAND FUNCTION
Constraint-induced movement therapy and hand-arm intensive bimanual therapy are designed to improve functionality of the hands. In constraint-induced movement therapy, the dominant hand is constrained to encourage development and use of the nondominant hand in children with hemiplegia. Hand-arm intensive bimanual therapy has similar goals and tasks but encourages use of both hands. In a trial of children with hemiplegic cerebral palsy, both of these strategies have been shown to improve function, which was sustained six months after completion of therapy.41 Hand-arm intensive bimanual therapy may be more tolerable in children who are frustrated with constraint-induced movement therapy.
EQUINUS DEFORMITY
Equinus deformity causes the classic hyperplantar flexion of the ankle in people with cerebral palsy. See https://bit.ly/35bIAYe for a photo of equinus deformity. Small gains in dorsiflexion could theoretically improve gait. Some studies show that ankle orthotics can help increase lower-limb motion and strength, resulting in improved walking function and parent satisfaction.42 There is insufficient evidence to support upper-limb orthotics.43
IMPROVING RANGE OF MOTION
Serial casting (casting to progressively stretch against contracture) has historically been used in patients with cerebral palsy to improve range of motion. However, evidence demonstrating functional improvement from these short-term, small increases in range of motion is limited.34,44 Therefore, this previously routine treatment should be considered only after other therapies fail.
Managing Associated Conditions
PRESSURE ULCERS
Preventing pressure ulcers is necessary for any patient with limited mobility, including those with cerebral palsy. Different positioning strategies, support surfaces, and prophylactic dressings should be used for at-risk individuals. Use of alternating pressure mattresses, wheelchair cushions, or medical-grade sheepskins for areas of pressure or friction should also be considered.18 Wound care consultation is recommended for recurrent or persistent pressure ulcers.
OSTEOPOROSIS
Osteoporosis is common in patients with cerebral palsy, likely a result of poor growth and nutrition, non–weight bearing status, limited exposure to sunlight, late-onset puberty, and use of anticonvulsants. It is estimated that 80% to 90% of children with cerebral palsy have low bone density and are at increased risk of fractures, most commonly in the femur.20
In patients 18 years or older, the Fracture Risk Assessment Tool or the QFracture tool can be used to determine whether a patient’s risk of osteoporosis merits treatment. If the patient is at high risk, dual energy x-ray absorptiometry can confirm the diagnosis of osteoporosis before starting treatment.19 Calcium and vitamin D supplements and bisphosphonates have been shown to improve bone density and reduce fracture rates.20
BEHAVIORAL, EMOTIONAL, AND PSYCHIATRIC ISSUES
Up to one in four children with cerebral palsy have behavioral or emotional issues. Many also meet criteria for comorbid psychiatric diagnoses, such as attention-deficit/hyperactivity disorder, conduct disorders, anxiety, and depression.45 Evaluation for these conditions is recommended to assure early access to resources and associated treatments.45 One treatment, cognitive behavior therapy, is designed to help patients identify and restructure negative thoughts and behaviors. Cognitive behavior therapy has been shown to be helpful in modifying behavior and managing emotions for a wide range of physical and mental conditions, although studies of patients with cerebral palsy are limited.
Continuing Care
Much of the research on cerebral palsy focuses on children and adolescents. However, most individuals with mild to moderate cases have near-normal life expectancies. For adults with cerebral palsy, it is important to consider the increased risk of secondary conditions as a result of a sedentary lifestyle, such as obesity, lower fitness, decreased bone density, and generally reduced functional reserve.46 Unless cerebral palsy–specific screening guidance is available, adolescents and adults with cerebral palsy should be assessed for chronic diseases, offered guidance on reproductive health, and screened for malignancies as indicated by the U.S. Preventive Services Task Force.
All members of the care team should address barriers to care, such as ensuring accessibility of buildings and availability of appropriate equipment (wheelchairs, hoists, bathroom items), facilitating transportation, addressing communication difficulties, offering longer appointments, and assisting patients in finding an advocate or support for social and emotional barriers to care. An annual evaluation with a neurodisability specialist is recommended for adults with GMFCS levels IV and V cerebral palsy.47
Prevention
Other than prevention of risk factors, there are few interventions known to reduce the risk of cerebral palsy. Although magnesium sulfate is not the standard initial treatment for premature labor, it has been shown to reduce the risk of cerebral palsy from 6.7% to 4.7% (relative risk = 0.68; number needed to treat = 48).48 There is some controversy as to whether antenatal steroids to promote fetal lung maturation in premature infants, particularly multiple courses, increase the risk of cerebral palsy. Therefore, the decision to initiate such therapy must be individualized based on potential benefits.13,49
Data Sources: A PubMed search was completed in Clinical Queries using the key terms cerebral palsy, motor function clinical assessments, treatment, medications, therapy, and comorbidities. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the American Academy of Neurology, National Institute for Health and Care Excellence, the Cochrane database, and Ovid MEDLINE. Search dates: December 8, 2018, and October 20, 2019.
The views expressed in this material are those of the authors and do not reflect the official policy or position of the U.S. government, the Department of Defense, or the Department of the Air Force.