Am Fam Physician. 2020;101(4):244-245
Author disclosure: No relevant financial affiliations.
Dupilumab (Dupixent) is a monoclonal antibody injection labeled for the treatment of moderate to severe asthma in patients 12 years and older with an eosinophilic phenotype or who require oral corticosteroid treatment. It was previously labeled for the treatment of atopic dermatitis and chronic rhinosinusitis with nasal polyposis.
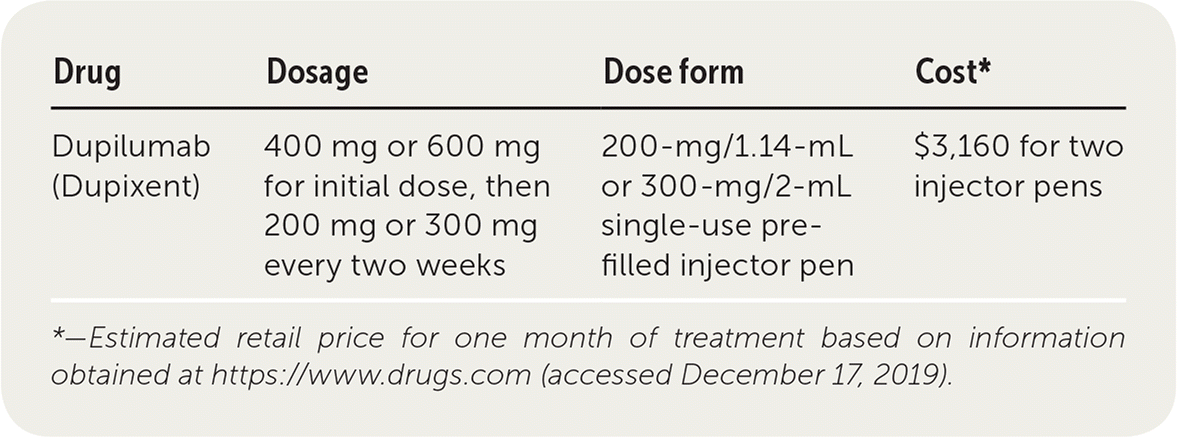
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Dupilumab (Dupixent) | 400 mg or 600 mg for initial dose, then 200 mg or 300 mg every two weeks | 200-mg/1.14-mL or 300-mg/2-mL single-use prefilled injector pen | $3,160 for two injector pens |
Safety
Hypersensitivity reactions occur in less than 1% of patients. Dupilumab carries a risk of eosinophilia, which may lead to serious conditions such as eosinophilic pneumonia or vasculitis. In clinical trials, 1% to 2% of participants developed eosinophilia.1 Although there have been isolated reports of eosinophilic pneumonia, there has been no significant increase in the incidence of severe eosinophilic conditions such as pneumonia, vasculitis, or granulomatosis, though studies are limited. Dupilumab has not been studied in women who are pregnant or lactating. Patients should not receive live vaccines while being treated with dupilumab.1
Tolerability
Dupilumab is generally well tolerated. The most common adverse effects are injection site reaction (10% to 14%), conjunctivitis (2% to 10%), oral herpes virus infection (4%), arthralgias (3%), and gastritis (2%). The rate of treatment discontinuation because of adverse effects was low in all available studies. Only 3% of participants discontinued treatment at the 200-mg dose compared with 6% of those taking the 300-mg dose and 4% in the placebo group.1
Effectiveness
In patients with moderate to severe asthma, one year of dupilumab therapy decreases the rate of severe asthma exacerbations (i.e., a worsening respiratory status requiring treatment for three or more days with a systemic glucocorticoid, hospitalization, or an emergency department visit leading to oral glucocorticoid use). Industry-supported trials included 2,888 patients who used a medium- or high-dose inhaled corticosteroid and up to two additional controller medications. Trial participants also had a forced expiratory volume in one second of less than 80% of predicted and worsening asthma control in the 12 months preceding enrollment as evidenced by emergency department or inpatient facility care requiring an oral steroid.1–3 Of these, 210 patients had oral corticosteroid–dependent asthma despite the use of a high-dose inhaled corticosteroid and two inhaled controller medications.3
After one year of twice-monthly injections, dupilumab decreased severe asthma exacerbations by 47.7% with the 200-mg dose and 46.0% with the 300-mg dose (i.e., approximately one fewer severe exacerbation over two and a half years) when added to existing therapy vs. placebo. Reductions in exacerbation were greater in patients with higher eosinophil counts. Compared with placebo, dupilumab decreased exacerbations in this population by 65.8% with the 200-mg dose and 67.4% with the 300-mg dose. Over 24 weeks, treatment with dupilumab lessened the need for oral glucocorticoid therapy, with 69% of patients weaning to a dosage of less than 5 mg daily (number needed to treat = 3) and 48% weaning off the medication entirely (number needed to treat = 4). Dupilumab has been compared with placebo but has not been compared with other controller medications or monoclonal antibodies.3
Price
Dupilumab is an expensive medication with variable insurance coverage. A one-month supply (two injector pens) costs about $3,160.
Simplicity
Dupilumab is a single-use, prefilled injector pen for subcutaneous injection every other week. The injections should be administered in the abdomen and thighs, rotating among sites, taking care to avoid the area 2 inches above the navel. The prefilled syringes must be stored in a refrigerator and allowed to come to room temperature for 30 to 45 minutes before use. Patients may self-administer the injection after receiving the proper training.1
Bottom Line
Dupilumab is an effective injectable drug that decreases asthma exacerbations and the need for an oral glucocorticoid in patients with moderate to severe asthma, particularly those with high eosinophil counts. However, it is expensive, and trials have only followed patients for up to one year and have not compared dupilumab with other monoclonal antibodies. Dupilumab is a well-tolerated add-on treatment for patients whose symptoms are not well controlled despite treatment with an inhaled corticosteroid and two other controller inhalers. Because of the high cost of dupilumab, primary care physicians should consider referral for phenotype testing and consultation before prescribing it.