Am Fam Physician. 2020;101(8):489-492
Related Putting Prevention into Practice: Screening for Asymptomatic Bacteriuria in Adults
As published by the USPSTF.
Summary of Recommendations
The USPSTF recommends screening for asymptomatic bacteriuria using urine culture in pregnant persons. B recommendation. The USPSTF recommends against screening for asymptomatic bacteriuria in nonpregnant adults (Table 1). D recommendation.
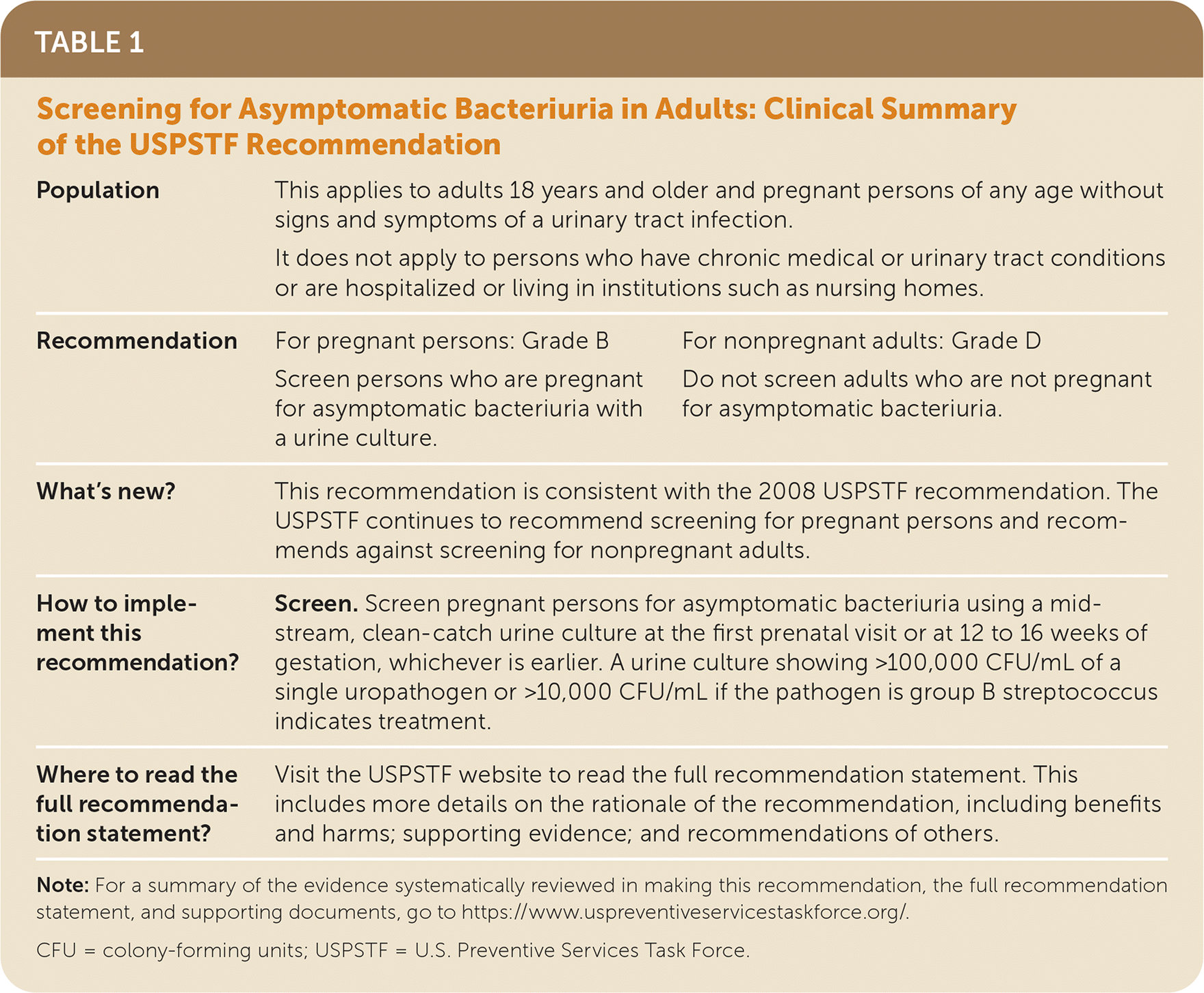
| Population | This applies to adults 18 years and older and pregnant persons of any age without signs and symptoms of a urinary tract infection. | |
| It does not apply to persons who have chronic medical or urinary tract conditions or are hospitalized or living in institutions such as nursing homes. | ||
| Recommendation | For pregnant persons: Grade B | For nonpregnant adults: Grade D |
| Screen persons who are pregnant for asymptomatic bacteriuria with a urine culture. | Do not screen adults who are not pregnant for asymptomatic bacteriuria. | |
| What's new? | This recommendation is consistent with the 2008 USPSTF recommendation. The USPSTF continues to recommend screening for pregnant persons and recommends against screening for nonpregnant adults. | |
| How to implement this recommendation? | Screen. Screen pregnant persons for asymptomatic bacteriuria using a midstream, clean-catch urine culture at the first prenatal visit or at 12 to 16 weeks of gestation, whichever is earlier. A urine culture showing >100,000 CFU/mL of a single uropathogen or >10,000 CFU/mL if the pathogen is group B streptococcus indicates treatment. | |
| Where to read the full recommendation statement? | Visit the USPSTF website to read the full recommendation statement. This includes more details on the rationale of the recommendation, including benefits and harms; supporting evidence; and recommendations of others. | |
Introduction
Asymptomatic bacteriuria is defined as the presence of bacteria in the urine of a person without signs or symptoms of a urinary tract infection.1 Among the general adult population, women (across all ages) have the highest prevalence of asymptomatic bacteriuria, although rates increase with age among both men and women.2 The reported prevalence of asymptomatic bacteriuria ranges from 1% to 6% among premenopausal women to 22% among women older than 90 years.3,4 Asymptomatic bacteriuria is present in an estimated 2% to 10% of pregnant women.5 The condition is rare in men.4,6
During pregnancy, physiologic changes that affect the urinary tract increase the risk of asymptomatic bacteriuria and symptomatic urinary tract infections, including pyelonephritis (a urinary tract infection in which one or both kidneys become infected).7 Pyelonephritis is one of the most common nonobstetric reasons for hospitalization in pregnant women.8 Pyelonephritis is associated with perinatal complications, including septicemia, respiratory distress, low birth weight, and spontaneous preterm birth.9
USPSTF Assessment of Magnitude of Net Benefit
PREGNANT PERSONS
The USPSTF concluded with moderate certainty that screening for and treatment of asymptomatic bacteriuria in pregnant persons have moderate net benefit in reducing perinatal complications (Table 2). There is adequate evidence that pyelonephritis in pregnancy is associated with negative maternal outcomes and that treatment of screen-detected asymptomatic bacteriuria can reduce the incidence of pyelonephritis in pregnant persons. However, evidence shows that the incidence of pyelonephritis among pregnant women with untreated asymptomatic bacteriuria has been low in recent decades, which may reduce the potential benefit from screening asymptomatic bacteriuria. When direct evidence is limited, absent, or restricted to select populations or clinical scenarios, the USPSTF may place conceptual upper or lower bounds on the magnitude of benefit or harms. Therefore, the USPSTF bounds the benefits of screening for asymptomatic bacteriuria in pregnant persons as no greater than moderate.
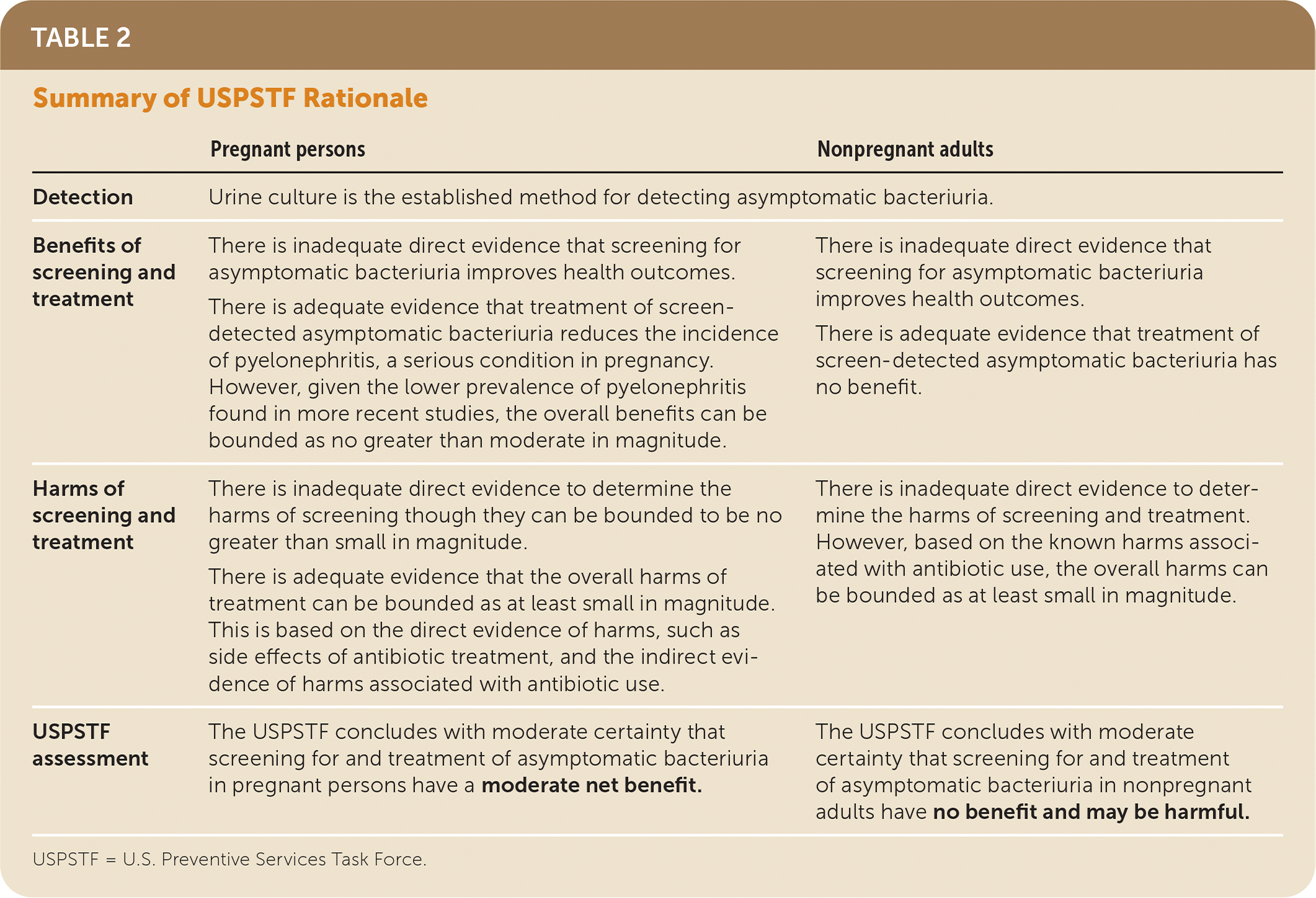
| Pregnant persons | Nonpregnant adults | |
|---|---|---|
| Detection | Urine culture is the established method for detecting asymptomatic bacteriuria. | |
| Benefits of screening and treatment | There is inadequate direct evidence that screening for asymptomatic bacteriuria improves health outcomes. There is adequate evidence that treatment of screen-detected asymptomatic bacteriuria reduces the incidence of pyelonephritis, a serious condition in pregnancy. However, given the lower prevalence of pyelonephritis found in more recent studies, the overall benefits can be bounded as no greater than moderate in magnitude. | There is inadequate direct evidence that screening for asymptomatic bacteriuria improves health outcomes. There is adequate evidence that treatment of screen-detected asymptomatic bacteriuria has no benefit. |
| Harms of screening and treatment | There is inadequate direct evidence to determine the harms of screening though they can be bounded to be no greater than small in magnitude. There is adequate evidence that the overall harms of treatment can be bounded as at least small in magnitude. This is based on the direct evidence of harms, such as side effects of antibiotic treatment, and the indirect evidence of harms associated with antibiotic use. | There is inadequate direct evidence to determine the harms of screening and treatment. However, based on the known harms associated with antibiotic use, the overall harms can be bounded as at least small in magnitude. |
| USPSTF assessment | The USPSTF concludes with moderate certainty that screening for and treatment of asymptomatic bacteriuria in pregnant persons have a moderate net benefit. | The USPSTF concludes with moderate certainty that screening for and treatment of asymptomatic bacteriuria in nonpregnant adults have no benefit and may be harmful. |
The USPSTF found inadequate direct evidence on the harms of screening for asymptomatic bacteriuria in pregnant persons, although these harms are thought to be no greater than small in magnitude. The USPSTF found adequate evidence of harms associated with treatment of asymptomatic bacteriuria, including adverse effects of antibiotic treatment. It also considered the potential effects of changes in the microbiome resulting from antibiotic use. Therefore, the USPSTF bounds the overall magnitude of harms of screening for asymptomatic bacteriuria in pregnant persons to be at least small.
NONPREGNANT ADULTS
The USPSTF concludes with moderate certainty that screening for and treatment of asymptomatic bacteriuria in nonpregnant adults have no net benefit (Table 2). There is adequate evidence that treatment of screen-detected asymptomatic bacteriuria in nonpregnant adults has no benefit. Based on the harms associated with antibiotic use, the USPSTF found adequate evidence to bound the harms of treatment of screen-detected asymptomatic bacteriuria in nonpregnant adults as at least small.
Practice Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to adults 18 years and older and pregnant persons of any age without signs and symptoms of a urinary tract infection. It does not apply to persons who have chronic medical or urinary tract conditions, such as end-stage renal disease; have indwelling urinary catheters, urinary stents, or spinal cord injuries; are hospitalized; reside in an institution (e.g., a nursing home); or are transplant recipients.
DEFINITION OF ASYMPTOMATIC BACTERIURIA
Asymptomatic bacteriuria occurs when the urinary tract is colonized with significant amounts of pathogenic bacteria, primarily from the gastrointestinal tract, in the absence of symptoms or signs of a urinary tract infection. The most common pathogen is Escherichia coli, although other bacteria such as Klebsiella, Proteus mirabilis, and group B streptococcus can be involved.4,11
ASSESSMENT OF RISK
The risk of developing asymptomatic bacteriuria varies by age, sex, and pregnancy status.6 Because of the location and length of the female urethra, women are predisposed to infections of the urinary tract, including asymptomatic bacteriuria.5 Physiologic changes in both pregnant and older women increase the risk of asymptomatic bacteriuria and a urinary tract infection.7,11,12 In general, men are at low risk of developing asymptomatic bacteriuria, although rates increase with older age.12 Persons with diabetes are also at increased risk of developing asymptomatic bacteriuria.4,6
SCREENING TESTS
Screening for asymptomatic bacteriuria during pregnancy is done with a urine culture at 12 to 16 weeks of gestation or at the first prenatal visit. Urine culture is currently recommended for screening in pregnancy and is the established method for diagnosis.2 A culture obtained using a midstream, clean-catch urine sample with greater than 100,000 colony-forming units per milliliter of a single uropathogen is considered a positive test result.6 Greater than 10,000 colony-forming units per milliliter of group B streptococcus is an indicator of vaginal colonization and is commonly used as the threshold for treatment of infection in pregnancy.13
SCREENING INTERVALS
In general, screening is performed once at the first prenatal visit per clinical guidelines. However, there is little evidence on the optimal timing and frequency of screening for asymptomatic bacteriuria in pregnancy.2
TREATMENT OR INTERVENTIONS
Pregnant persons with asymptomatic bacteriuria usually receive antibiotic therapy, based on urine culture results and follow-up monitoring. The choice of antibacterial regimen for treatment of asymptomatic bacteriuria during pregnancy is based on safety in pregnancy and patterns of antimicrobial resistance in the particular setting.6,7
This recommendation statement was first published in JAMA. 2019;322(12):1188–1194.
The “Update of Previous USPSTF Recommendation,” “Supporting Evidence,” “Research Needs and Gaps,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/asymptomatic-bacteriuria-in-adults-screening#fullrecommendationstart.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.