Am Fam Physician. 2020;102(2):115-116
Author disclosure: No relevant financial affiliations.
Dapagliflozin (Farxiga) is a selective sodium-glucose cotransporter 2 inhibitor that promotes glycosuria. It is labeled for the treatment of type 2 diabetes mellitus in adults as an adjunct to diet and exercise to improve glycemic control. Recently the labeling has been revised to include use in reducing the risk of hospitalization for heart failure in patients with type 2 diabetes and established cardiovascular disease (CVD) or multiple cardiovascular risk factors, and to reduce the risk of cardiovascular death and hospitalization of adults with New York Heart Association (NYHA) class II to IV heart failure with reduced ejection fraction (HFrEF), with or without type 2 diabetes.
Safety
In postmarketing reports of dapagliflozin, adverse effects include hypotension, hypoglycemia, ketoacidosis, acute kidney injury, urosepsis, pyelonephritis, and necrotizing fasciitis of the perineum.1 Patients with diabetes who have or are at high risk of CVD are more likely to develop ketoacidosis (number needed to harm [NNH] = 500).2 Dapagliflozin is not recommended for use in patients with hypovolemia or those with an estimated glomerular filtration rate (eGFR) of less than 45 mL per minute per 1.73 m2. It should not be prescribed to patients with an eGFR of less than 30 mL per minute per 1.73 m2, those with end-stage renal disease, pregnant women in the second and third trimesters, or lactating women.1
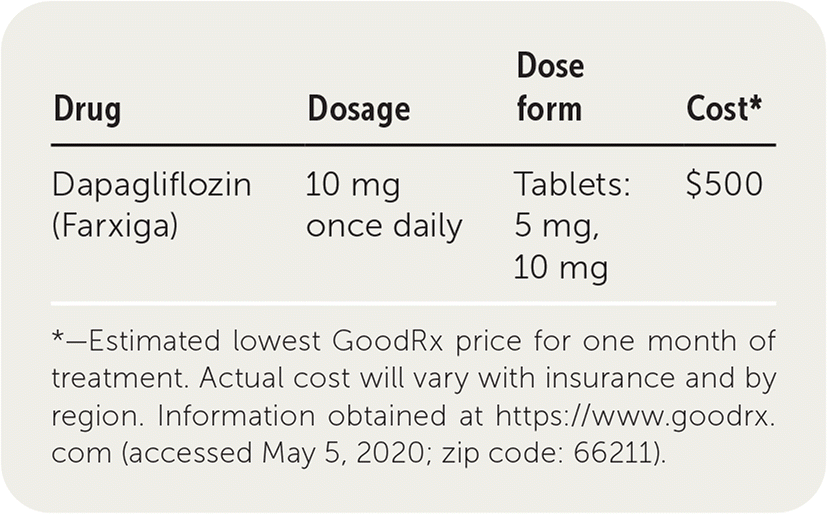
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Dapagliflozin (Farxiga) | 10 mg once daily | Tablets: 5 mg, 10 mg | $500 |
Tolerability
Dapagliflozin is well tolerated in patients who have HFrEF, with a drop-out rate similar to that of placebo (4.7%).3 About 8% of patients with diabetes who also have CVD or risk factors for CVD will stop treatment because of adverse effects (NNH = 83). These patients are more likely to develop genital yeast infections (NNH = 125) while taking dapagliflozin compared with patients taking placebo.2
Effectiveness
Dapagliflozin has been shown to reduce the risk of hospitalization for heart failure in patients with type 2 diabetes who have or are at high risk of CVD (men older than 55 years or women older than 60 years with hypertension, hyperlipidemia, smoking, or statin use). There were fewer hospitalizations for heart failure in patients taking dapagliflozin compared with patients taking placebo over the median 4.2-year study period (2.5% vs. 3.3%; number needed to treat [NNT] = 113; 95% CI, 78 to 256). Patients taking dapagliflozin were less likely to experience the combined renal endpoint of a greater than 40% decrease in eGFR (less than 60 mL per minute per 1.73 m2), development of end-stage renal disease, or death from a renal or cardiovascular cause compared with patients taking placebo (14.1% vs. 10.8%; NNT = 77; 95% CI, 56 to 141). The primary benefit was from a reduction in renal disease progression and death from renal causes. However, because there was no difference in the rate of major adverse cardiovascular events (i.e., cardiovascular-related death, myocardial infarction, or ischemic stroke) between patients taking dapagliflozin and placebo, trial investigators consider the reduction in combined renal outcome as hypothesis-generating.2
Dapagliflozin reduces the risk of worsening HFrEF or death from a cardiovascular cause in patients with HFrEF with an ejection fraction of 40% or less. When compared with placebo in patients with NYHA class II to IV HFrEF, who also received standard medical and device treatment, dapagliflozin reduced rates of worsening HFrEF or cardiovascular-related death from 21.2% to 16.3% over the 18.2-month study period (NNT = 20; 95% CI, 15 to 35).3 Patients with and without diabetes benefited from treatment.
Price
A one-month supply of dapagliflozin costs approximately $500. It is similar in price to empagliflozin (Jardiance), which is also labeled for cardiovascular event reduction in patients with diabetes and CVD and costs about $505 for a 30-day supply. Approximately 50% of Medicare Part D plans provide coverage for dapagliflozin.
Simplicity
The recommended dosage of dapagliflozin is 10 mg taken once daily to reduce the risk of hospitalization from heart failure or cardiovascular death. eGFR should be measured before starting treatment and periodically thereafter. Patients should also be monitored for hypotension.
Bottom Line
Dapagliflozin can be added to existing treatment to reduce the risk of hospitalization for heart failure in patients with type 2 diabetes who have established CVD or are at high risk of CVD. It may also decrease the risk of renal disease progression and death from renal failure. Dapagliflozin can be added to existing HFrEF treatment to reduce hospitalization and death from cardiovascular causes in patients with heart failure with or without type 2 diabetes.3