
Am Fam Physician. 2020;102(7):online
Author disclosure: No relevant financial affiliations.
Details for This Review
Study Population: Women who underwent operative vaginal delivery
Efficacy End Points: Reduction in infectious postpartum morbidities such as wound infection, wound breakdown, endometritis, and sepsis in women with less than a third-degree tear (antibiotics are commonly administered for third- or fourth-degree tears)
Harm End Points: Adverse reaction to antibiotic therapy
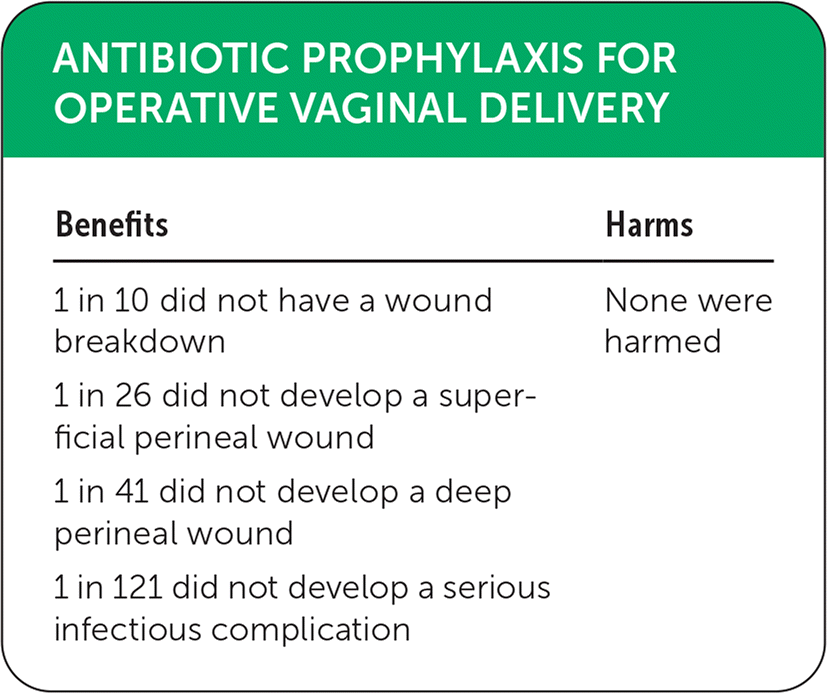
| Benefits | Harms |
|---|---|
| 1 in 10 did not have a wound breakdown | None were harmed |
| 1 in 26 did not develop a superficial perineal wound | |
| 1 in 41 did not develop a deep perineal wound | |
| 1 in 121 did not develop a serious infectious complication |
Narrative: Operative vaginal birth is used to achieve or expedite safe delivery for maternal or fetal indications, and is accomplished using traction on the fetal head through the application of forceps or a vacuum extractor.1 Despite success in achieving vaginal birth, operative vaginal deliveries may result in an increased incidence of postpartum infections and maternal readmissions when compared with spontaneous vaginal deliveries.2 Despite these potential complications, current guidelines from the World Health Organization do not recommend routine antibiotic prophylaxis for operative vaginal birth because of insufficient evidence of effectiveness.3
This Cochrane review included two randomized controlled trials with a total of 3,813 pregnant women undergoing operative vaginal delivery using a vacuum or forceps.2
The ANODE trial was a blinded, randomized, multicenter trial conducted in the United Kingdom that consisted of 3,420 women. The trial compared a single dose of intravenous amoxicillin/clavulanate (Augmentin) with placebo.2 The other study involved 393 women in the United States and compared intravenous cefotetan (Cefotan) with no treatment.4
Benefits of antibiotic therapy included a reduction in superficial (epidermis only) perineal wounds (relative risk [RR] = 0.53; 95% CI, 0.40 to 0.69; number needed to treat [NNT] = 26; high-certainty evidence); reduction in deep (muscle/fascial involvement) perineal wounds (RR = 0.46; 95% CI, 0.31 to 0.69; NNT = 41; high-certainty evidence); reduction in serious infectious complications (RR = 0.44; 95% CI, 0.22 to 0.89; NNT = 121; high-certainty evidence); and reduction in wound breakdown (RR = 0.52; 95% CI, 0.43 to 0.63; NNT = 10; moderate-certainty evidence).
The effects of prophylactic antibiotics on organ or space infection, endometritis, and length of hospitalization were unclear secondary to low-certainty evidence. The adverse events were minimal in both studies and included two allergic reactions (neither of which were anaphylactic; one was considered a serious adverse event) and diarrhea.
Secondary outcomes that were evaluated, but with inconclusive results, included perineal pain, dyspareunia, additional perineal care requirements, use of pain medications, effect on breastfeeding, hospital readmission, and quality of life.
Caveats: The exclusion criteria included patients requiring antibiotic therapy in the postpartum period for another indication, most notably third- and fourth-degree lacerations. Additional studies may be indicated to compare this subset of patients because the use of prophylactic antibiotics for this classification of perineal trauma is not standardized. Additionally, the definition of endometritis between the included studies was different and may have affected the determination of the certainty of the evidence. The prevalence of wound breakdown was markedly elevated in the ANODE trial (21%); this may have been affected by self-reported data and the absence of a uniform wound breakdown definition or criteria.5 Typical wound breakdown rates are 5.5% for forceps and 1.4% for vacuum delivery based on two recent cohort studies (n = 529, P < .01).6
In this review, the largest sample of patients (ANODE trial) used an antibiotic formulation not readily available in the United States. Therefore, the effect of using alternative antibiotics may be an area for further evaluation. Additionally, the larger trial was conducted in a high-income setting, limiting the generalizability across a more diverse socioeconomic demographic.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Air Force, the Uniformed Services University of the Health Sciences, the Department of Defense, or the U.S. government.
Copyright © 2020 MD Aware, LLC (theNNT.com). Used with permission.
This series is coordinated by Christopher W. Bunt, MD, AFP assistant medical editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
