
Am Fam Physician. 2021;103(1):online
Author disclosure: No relevant financial affiliations.

Details for This Review
Study Population: Children five to 18 years of age with nocturnal enuresis
Efficacy End Points: Percentage of children achieving 14 consecutive dry nights at treatment conclusion (primary outcome), percentage of children maintaining 14 consecutive dry nights two weeks to 31 months after treatment conclusion (secondary outcome)
Harm End Points: Percentage of children with adverse events, including the alarm failing to wake up the child, ringing without urination, waking others, frightening the child, causing discomfort, or being too difficult to use (secondary outcome)
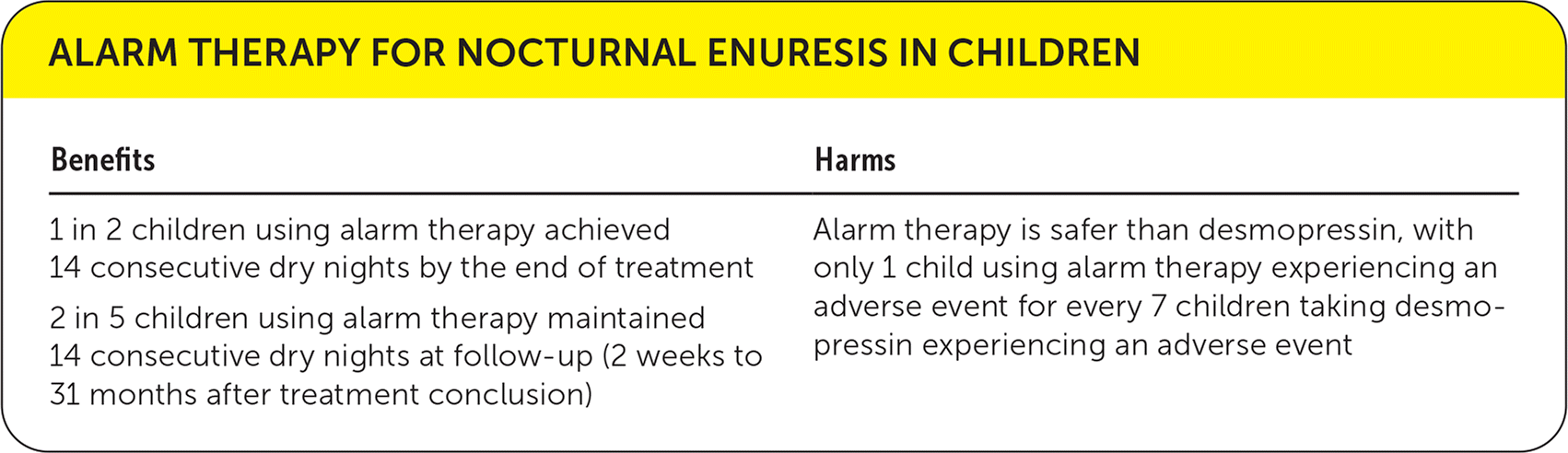
| Benefits | Harms |
|---|---|
| 1 in 2 children using alarm therapy achieved 14 consecutive dry nights by the end of treatment | Alarm therapy is safer than desmopressin, with only 1 child using alarm therapy experiencing an adverse event for every 7 children taking desmopressin experiencing an adverse event |
| 2 in 5 children using alarm therapy maintained 14 consecutive dry nights at follow-up (2 weeks to 31 months after treatment conclusion) |
Narrative: Nocturnal enuresis is common in children, affecting up to 20% of five-year-olds, 7.75% of eight-year-olds, 3% of 11-year-olds, and 0.7% of 17-year-olds.1–3 There is an annual spontaneous remission rate of 14% to 15% during childhood and adolescence.4 Although it is a pathologically benign condition with a high rate of spontaneous remission, it can affect a child's quality of life and self-esteem and impact social, emotional, and psychological well-being. It is important to identify effective interventions for enuresis. This review assessed the effects of alarm therapy for treating nocturnal enuresis in children, compared with other interventions.1
The systematic review included 74 randomized or quasirandomized controlled trials, involving a total of 5,983 children. The trials compared alarm therapy with medications, placebo, behavioral interventions, control treatments (e.g., nonfunctioning alarms, an alarm that rang in the parents' room 20 minutes after triggering, a wait list, taking the child to the toilet twice a night), or alarms used in combination with other treatments. Primary outcomes included average number of wet nights per week and the percentage of children with 14 consecutive dry nights at the end of treatment. Secondary outcomes were similar but assessed at subsequent follow-up (two weeks to 31 months after treatment conclusion). The review also investigated adverse effects of alarm therapy compared with other treatments as a secondary outcome.
Compared with no treatment or a control intervention, alarm therapy reduced the average number of wet nights per week by 3.4 days (95% CI, 1.87 to 4.98; four studies; 100 children; low-quality evidence), and children were more likely to attain 14 consecutive dry nights at treatment conclusion (relative risk [RR] = 7.23; 95% CI, 1.40 to 37.33; 18 studies; 827 children; number needed to treat [NNT] = 1.94; low-quality evidence). Similarly, alarm therapy was superior to placebo in terms of attaining 14 consecutive dry nights at treatment conclusion (RR = 1.59; 95% CI, 1.16 to 2.17; two studies; 181 children; NNT = 3.67; low-quality evidence). Compared with no treatment or a control intervention, children using alarm therapy were also more likely to maintain 14 consecutive dry nights at follow-up (RR = 9.67; 95% CI, 4.74 to 19.76; 10 studies; 366 children; NNT = 2.45; low-quality evidence).1
Many of the comparisons between alarm therapy and other treatments did not achieve statistical significance, although alarm therapy showed statistical superiority in achieving 14 consecutive dry nights at treatment conclusion compared with tricyclic antidepressants (RR = 2.05; 95% CI, 1.33 to 3.17; three studies; 208 children; NNT = 4.65; very low-quality evidence), psychotherapy (RR = 3.62; 95% CI, 1.38 to 9.50; two studies; 116 children; NNT = 1.85; very low-quality evidence), hypnotherapy (RR = 3.00; 95% CI, 1.18 to 7.60; one study; 96 children; NNT = 3.43; very low-quality evidence), or a restricted diet (RR = 23.00; 95% CI, 3.19 to 165.98; one study; 150 children; NNT = 3.41; very low-quality evidence). Alarm therapy also had a lower risk of adverse events compared with desmopressin (RR = 0.38; 95% CI, 0.20 to 0.71; five studies; 565 children; moderate-quality evidence).1
Caveats: This review is limited by low-quality evidence. Despite including a large number of trials, many trials did not report data in a usable way, and most did not report or mention adverse effects. Most of the trials were small, with 30 studies including 50 or fewer participants. Most of the comparisons analyzed in the review included only one or two trials, and the low-quality evidence limited the strength of the conclusions. Because of poor reporting of study methods in most trials, it is difficult to assess study bias, and none were judged to be at low risk of bias overall.1
Conclusion: The review concluded that alarm therapy appears to be effective for treating nocturnal enuresis in children; however, because of risk of bias and underpowered analyses, it is uncertain if alarm therapy is more effective than many of the other interventions used for enuresis. Further studies should focus on study designs that minimize bias, strengthen the validity of comparisons between alarms and other treatments, and use larger sample sizes.1
