
Am Fam Physician. 2021;103(1):16-18
Author disclosure: No relevant financial affiliations.
Clinical Question
Is augmentation with a second antidepressant or an atypical antipsychotic effective for treatment-resistant depression in adults?
Evidence-Based Answer
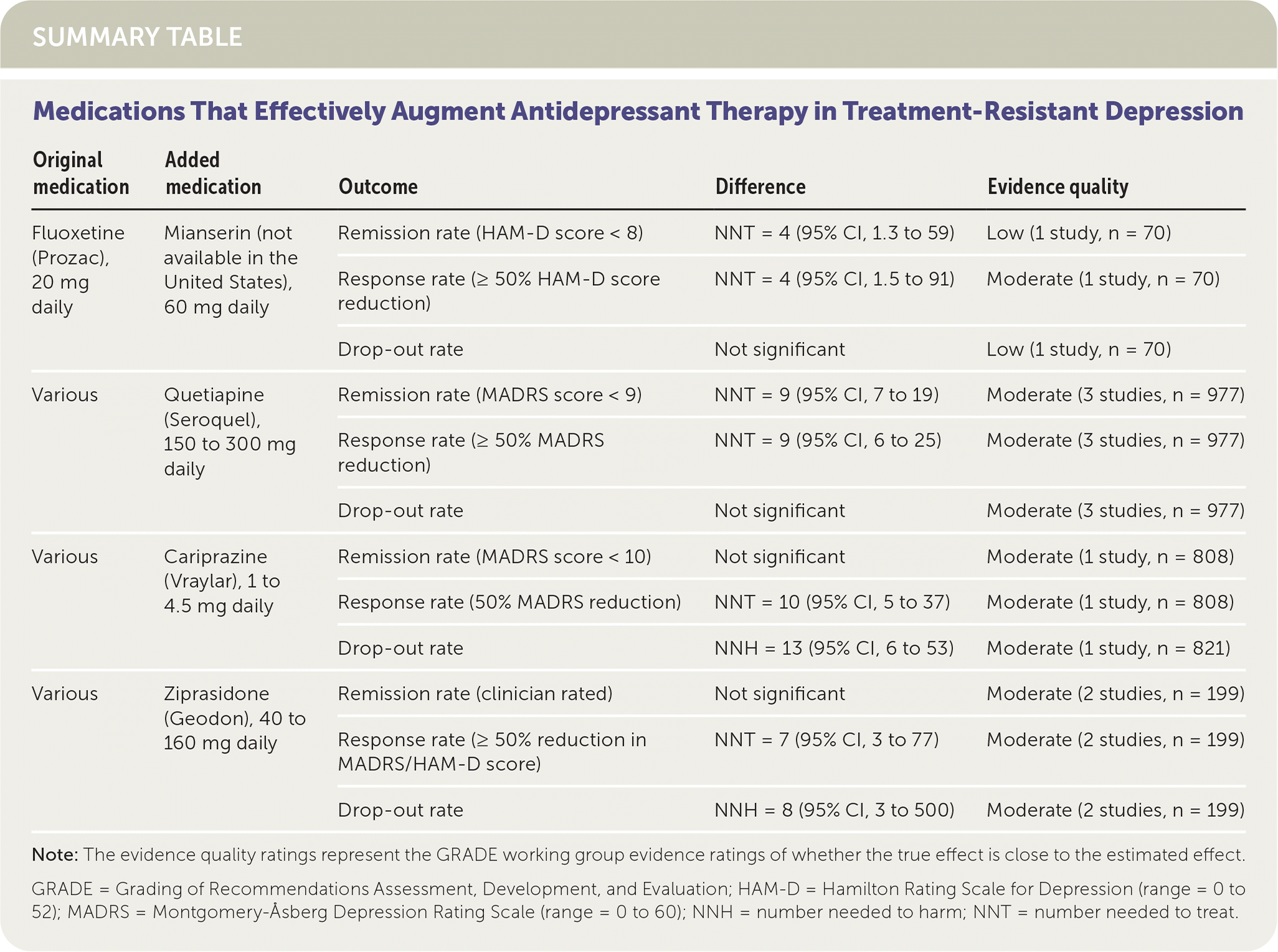
In patients with treatment-resistant depression, augmenting therapy with atypical antipsychotics can be effective. Adding quetiapine (Seroquel) to antidepressant therapy reduces symptoms below the remission threshold (number needed to treat [NNT] = 9), whereas the number of people who stop using the medicine (dropouts) increases only at the highest dosage. Augmentation with cariprazine (Vraylar) or ziprasidone (Geodon) improves the clinical response; however, the benefit is offset by increased dropouts.1 (Strength of Recommendation: C, based on disease-oriented outcomes.)
Practice Pointers
Treatment-resistant depression is defined as failure to respond to one or more antidepressant medications at therapeutic doses and occurs in at least 12% of patients with depression.1,2 In a large major depression trial, treatment resistance was observed in one-third of patients.3 Only 20% of patients with treatment resistance achieve remission, even after multiple interventions.2 Patients with treatment-resistant depression also have a 17% rate of attempted suicide and report suicidal ideation twice as often as those with treatment-responsive depression.2
This Cochrane review included 10 randomized controlled trials with 2,731 patients.1 Nine studies were industry funded and conducted in the outpatient setting. One study involved switching medications, whereas the others augmented therapy with a second medication. The most common baseline antidepressant was fluoxetine (Prozac). Most studies had 12 weeks or less of follow-up, but one included follow-up at one year.
Because changes in average depression scores are difficult to interpret, studies reported more clinically oriented measures. The response rate was defined as the number of participants whose depression scores were reduced by at least 50% from baseline. The remission rate was defined as the number of participants with depression scores reduced below a threshold that demonstrates resolution of depression. Studies also reported dropout rates for any reason as a measure of treatment harm.
One small, low-quality study demonstrated that switching from fluoxetine to mianserin (a tetracyclic antidepressant not available in the United States) did not improve depression scores, response rates, or remission rates.
Two European studies evaluated the benefit of augmenting fluoxetine therapy with another antidepressant. Augmentation with mianserin at a daily dosage of 60 mg increased both response and remission rates, without increasing drop-out rates. Augmentation with mirtazapine (Remeron), 30 mg daily, failed to improve depression symptoms, response rates, or remission rates after 12, 24, or 52 weeks.
One study in which fluoxetine or citalopram (Celexa) was augmented with buspirone (Buspar), 10 to 30 mg twice daily, showed no improvement in depression scores or response rates compared with placebo.
Augmentation with atypical antipsychotics had more evidence of benefit. In three studies, augmenting tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or bupropion (Wellbutrin) with quetiapine, 150 to 300 mg daily, increased rates of clinical response (NNT = 9; 95% CI, 7 to 19) and remission (NNT = 9; 95% CI, 6 to 25). Although use of quetiapine did not affect drop-out rates overall, dosages of 300 mg daily increased dropouts, whereas lower dosages did not.
Augmenting an SSRI or SNRI with cariprazine, 1 to 4.5 mg daily, increased clinical response (NNT = 10; 95% CI, 5 to 37) in one moderate-quality study but did not increase the remission rate. Treatment with this agent increased the risk of dropout (number needed to harm [NNH] = 13; 95% CI, 6 to 53). In two studies, augmentation with ziprasidone, 40 to 160 mg daily, increased the response rate (NNT = 7; 95% CI, 3 to 77) with no effect on remission, but it also increased dropouts (NNH = 8; 95% CI, 3 to 500).
Guidelines from the Institute for Clinical Systems Improvement (ICSI) and the National Institute for Health and Care Excellence (NICE) recommend augmenting antidepressant therapy to manage treatment-resistant depression. ICSI suggests augmentation with bupropion, buspirone, mirtazapine, thyroxine, stimulants, lithium, or atypical antipsychotics.4 NICE suggests switching antidepressant medications or augmenting with lithium, an antipsychotic, or mirtazapine if the patient is willing to tolerate increased adverse effects.5
| Original medication | Added medication | Outcome | Difference | Evidence quality |
|---|---|---|---|---|
| Fluoxetine (Prozac), 20 mg daily | Mianserin (not available in the United States), 60 mg daily | Remission rate (HAM-D score < 8) | NNT = 4 (95% CI, 1.3 to 59) | Low (1 study, n = 70) |
| Response rate (≥ 50% HAM-D score reduction) | NNT = 4 (95% CI, 1.5 to 91) | Moderate (1 study, n = 70) | ||
| Drop-out rate | Not significant | Low (1 study, n = 70) | ||
| Various | Quetiapine (Seroquel), 150 to 300 mg daily | Remission rate (MADRS score < 9) | NNT = 9 (95% CI, 7 to 19) | Moderate (3 studies, n = 977) |
| Response rate (≥ 50% MADRS reduction) | NNT = 9 (95% CI, 6 to 25) | Moderate (3 studies, n = 977) | ||
| Drop-out rate | Not significant | Moderate (3 studies, n = 977) | ||
| Various | Cariprazine (Vraylar), 1 to 4.5 mg daily | Remission rate (MADRS score < 10) | Not significant | Moderate (1 study, n = 808) |
| Response rate (50% MADRS reduction) | NNT = 10 (95% CI, 5 to 37) | Moderate (1 study, n = 808) | ||
| Drop-out rate | NNH = 13 (95% CI, 6 to 53) | Moderate (1 study, n = 821) | ||
| Various | Ziprasidone (Geodon), 40 to 160 mg daily | Remission rate (clinician rated) | Not significant | Moderate (2 studies, n = 199) |
| Response rate (≥ 50% reduction in MADRS/HAM-D score) | NNT = 7 (95% CI, 3 to 77) | Moderate (2 studies, n = 199) | ||
| Drop-out rate | NNH = 8 (95% CI, 3 to 500) | Moderate (2 studies, n = 199) |
The practice recommendations in this activity are available at http://www.cochrane.org/CD010557.
Editor's Note: The NNTs, NNHs, and CIs reported in this Cochrane for Clinicians were calculated by the authors based on raw data provided in the original Cochrane review. Dr. Arnold is a contributing editor for AFP.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Air Force, Uniformed Services University of the Health Sciences, Department of Defense, or the U.S. government.
