
Am Fam Physician. 2021;103(11):646-647
Published online April 30, 2021.
Author disclosure: No relevant financial affiliations.
To the Editor: Nearly 7 million doses of Johnson & Johnson's Janssen (Ad26.COV2.S) viral vector vaccine have been administered in the United States to combat the COVID-19 pandemic. Several cases of vaccine-induced thrombotic thrombocytopenia from a similar viral vector vaccine (Oxford–AstraZeneca [ChAdOx1 nCoV-19]) have been reported.1,2 More recently, six cases of cerebral venous sinus thrombi in young women were reported after receiving the Janssen Ad26.COV2.S vaccine, which led the Centers for Disease Control and Prevention and the U.S. Food and Drug Administration to recommend temporarily pausing its use.3 We present a case of a patient with a cerebral venous sinus thrombosis, pulmonary embolism, and thrombocytopenia after receiving the Janssen Ad26.COV2.S vaccine.
A 40-year-old woman with a history of migraines, obesity, and no other known thrombotic risk factors developed a sudden headache, body aches, fever, and chills six days after receiving the Janssen vaccine. The patient presented to an urgent care facility on day 8 because of worsening pain with sinus pressure and was prescribed amoxicillin/clavulanate (Augmentin), methocarbamol (Robaxin), and methylprednisolone for presumed acute sinusitis. On day 9, her headache improved, but she developed swollen red cheeks and bilateral lower-extremity pain without edema. On day 10, her lower-extremity pain resolved, but her headache worsened, and she experienced intermittent vertigo. As the redness and swelling resolved, she noticed petechiae on her right cheek and bilateral breasts and spontaneous bruising in her extremities. On day 12, she presented to the emergency department for an intolerable headache. Laboratory and imaging studies demonstrated thrombocytopenia (platelets of 20,000), a d-dimer of 45,570 ng per mL (normal is less than 500 ng per mL), pulmonary emboli, and dural venous sinus thrombosis (Figure 1). An initial in-house heparin-induced thrombocytopenia antibody enzyme-linked immunosorbent assay (ELISA) test result was negative; however, a confirmatory heparin-induced thrombocytopenia test found a positive platelet factor-4 ELISA, serotonin release assay, and platelet P-selectin expression assay. Although this patient had no previous exposure to heparin, the clinical presentation and laboratory results were similar to heparin-induced thrombotic thrombocytopenia. This patient's presentation is consistent with the reported cases of suspected vaccine-induced thrombotic thrombocytopenia recently described in the literature.1,2,4
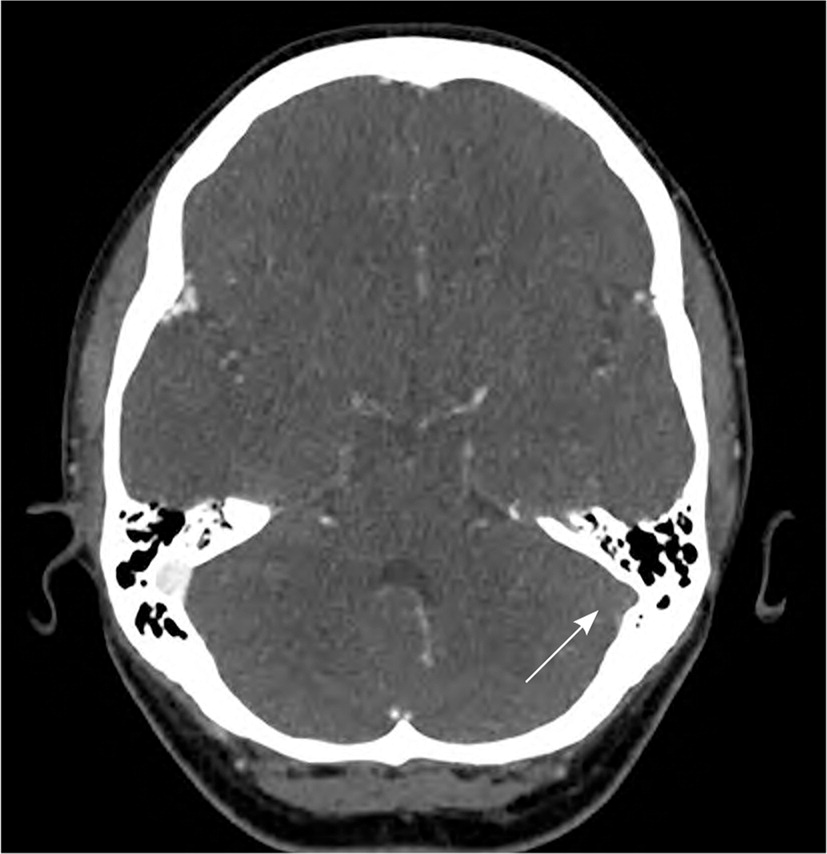
The patient was treated with a nonheparin anticoagulant, bivalirudin (Angiomax). She was started on prednisone, 1 mg per kg per day, and two days of intravenous immunoglobulin at 1 g per kg per day for thrombocytopenia. After the cerebral venous sinus thrombosis decreased in volume on subsequent imaging, she was discharged home on hospital day 7 on rivaroxaban (Xarelto) and a prednisone taper. At a follow-up visit, the platelet count had normalized and her symptoms had improved. Although these complications are currently extremely rare relative to the number of vaccines administered, understanding the responsible mechanism will be essential to determine individuals who are at higher risk of adverse effects from the viral vector COVID-19 vaccines.
Editor's Note: On April 23, 2021, the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention lifted their temporary pause on the use of the Ad26. COV2.S viral vector vaccine in the United States, concluding that “this vaccine is safe and effective in preventing COVID-19” and that “the vaccine’s known and potential benefits outweigh its known and potential risks in individuals 18 years of age and older.”1 After a thorough safety review, the agencies identified 15 cases of vaccine-induced thrombotic thrombocytopenia (including the original six cases that prompted the pause) among nearly 7 million doses administered before April 13, all in women between 18 and 59 years of age, with symptom onset six to 15 days postvaccination.1 In addition, the phase 3 trial data on the vaccine submitted by Janssen to the FDA for emergency use authorization were recently published, demonstrating a 67% to 77% efficacy against mild to severe COVID-19 at least 14 days after administration.2 The editors of AFP agree that the individual and public health benefits of continuing to administer the Janssen vaccine to our patients greatly outweigh the rare adverse effect documented in this case report.—Kenny Lin, MD, MPH, Deputy Editor
References
1. U.S. Food and Drug Administration. FDA and CDC lift recommended pause on Johnson & Johnson (Janssen) COVID-19 vaccine use following thorough safety review. April 23, 2021. Accessed April 24, 2021. https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough
2. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. April 21, 2021. Accessed April 24, 2021. https://www.nejm.org/doi/10.1056/NEJMoa2101544?url_ver=Z39.88-2003&rfr_id=ori: rid:crossref.org& rfr_dat= cr_pub%20%200pubmed
