
Am Fam Physician. 2021;103(11):688-690
Author disclosure: No relevant financial affiliations.
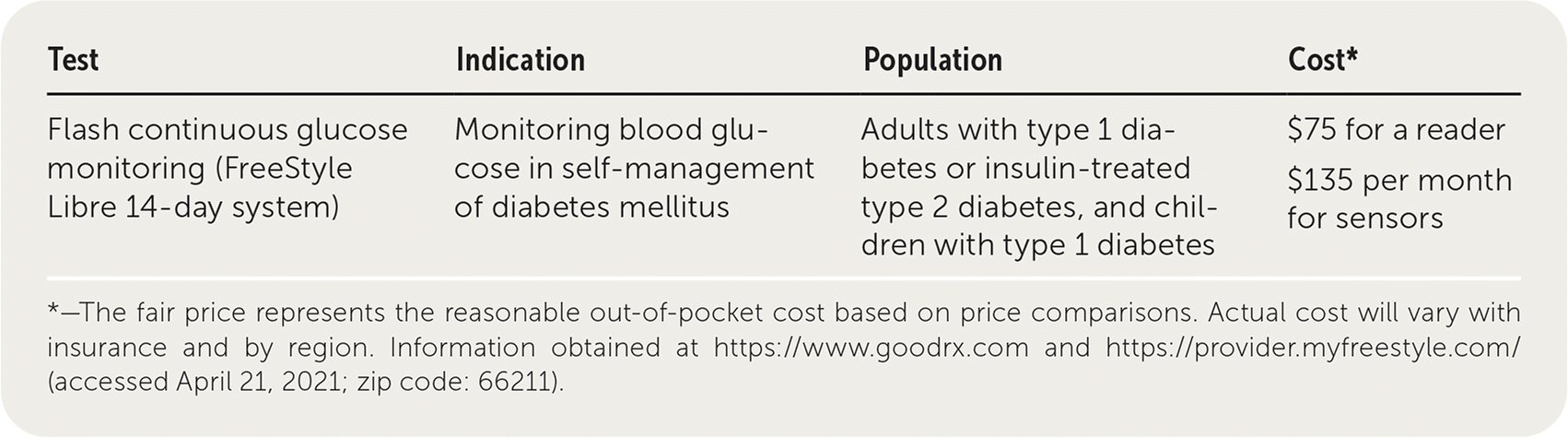
| Test | Indication | Population | Cost* |
|---|---|---|---|
| Flash continuous glucose monitoring (FreeStyle Libre 14-day system) | Monitoring blood glucose in self-management of diabetes mellitus | Adults with type 1 diabetes or insulin-treated type 2 diabetes, and children with type 1 diabetes | $75 for a reader $135 per month for sensors |
Continuous glucose monitors (CGMs) are used for the self-management of diabetes mellitus and have a subcutaneously inserted sensor that measures glucose in the interstitial fluid and transmits the result to a receiver. The FreeStyle Libre 14-day system is an intermittently scanned or “flash” CGM that was approved by the U.S. Food and Drug Administration in 2017. It displays glucose values when the sensor is scanned with the receiver. The sensor is placed on the posterior upper arm, lasts 14 days, and is factory calibrated. FreeStyle Libre displays eight-hour glucose trends but does not have any alarms.1,2 Real-time CGMs measure glucose every one to five minutes and issue alarms when glucose values are too high or low. Some brands may need to be calibrated by self-monitoring of capillary blood glucose (SMBG), which uses a finger stick and test strips.1,2
Accuracy
FreeStyle Libre is accurate in adults and children (mean absolute relative difference = 11.4% and 13.9%, respectively), with capillary blood glucose as the reference standard.3,4 Its accuracy is stable throughout the 14-day lifespan of the sensor but is lower during hypoglycemia and exercise and after a glucose load.3,5,6
Benefit
A systematic review and meta-analysis of 15 randomized controlled trials (RCTs) evaluated the impact of CGMs (including real-time and flash CGMs) on glycemic control compared with usual care using SMBG (n = 2,477).7 It included three studies of FreeStyle Libre in adults; two of the studies evaluated adults with type 1 diabetes and one study evaluated adults with insulin-treated type 2 diabetes. In general, CGMs produced a pooled A1C reduction of −0.17% (95% CI, −0.29% to −0.06%), greater time in euglycemic range (70.74 minutes per day; 95% CI, 46.73 to 94.76), less time in hyperglycemia (−30.26 minutes per day; 95% CI, −58.15 to −2.38), and less time in hypoglycemia (−27.16 minutes per day; 95% CI, −42.08 to −12.25). A subanalysis of the FreeStyle Libre trials (n = 626) found similar effects on time in euglycemic range, hyperglycemia, and hypoglycemia, but no statistically significant A1C reduction.
A meta-analysis of 12 studies (three RCTs, five prospective cohorts, three retrospective cohorts, one not reported; n = 2,173) evaluating the use of FreeStyle Libre in adults and children with type 1 diabetes or insulin-treated type 2 diabetes showed that the use of FreeStyle Libre reduced A1C levels by −0.26% (95% CI, −0.43% to −0.09%) compared with baseline. Compared with SMBG, however, A1C reduction was no longer significant.8
ADULTS WITH TYPE 1 DIABETES
An RCT of adults with well-controlled type 1 diabetes (n = 241) showed no A1C reduction in patients using FreeStyle Libre compared with SMBG.9 FreeStyle Libre was associated with increased time in euglycemic range (one hour per day), decreased hypoglycemic events (−0.45 events per day), decreased time in hypoglycemia (−1.24 hours per day), and decreased time in hyperglycemia (−0.37 hours per day).9
A prospective cohort study of FreeStyle Libre found a decrease in the number of people admitted to the emergency department or hospital for hypoglycemia or diabetic ketoacidosis (from 3.3% to 2.2%), number of people with severe hypoglycemic episodes requiring third-party help (14.6% to 7.8%), number of people with hypoglycemic comas (2.7% to 1.1%), and diabetes-related absenteeism (5.8% to 2.9%) at 12 months compared with baseline.10
Although both studies found statistically significant improvements in treatment satisfaction (Diabetes Treatment Satisfaction Questionnaire score) and perceived frequency of hyperglycemia compared with SMBG, there were no improvements in quality of life, Diabetes Distress Scale score, or hypoglycemia fear scores.9,10
CHILDREN WITH TYPE 1 DIABETES
A meta-analysis of 10 observational studies (n = 908) showed an A1C reduction of −0.29% (95% CI, −0.47% to −0.10%) from baseline in children with type 1 diabetes using FreeStyle Libre.11 In an RCT comparing FreeStyle Libre with SMBG (n = 51), there was no difference in time in euglycemic range, hypoglycemic time or events, or hyperglycemic time or events.12 A prospective cohort study of FreeStyle Libre found a significant decrease in the proportion of patients with severe hypoglycemic events at 12 months (6.8% to 3.2%) compared with baseline, whereas there was no significant decrease in SMBG users.13
ADULTS WITH INSULIN-TREATED TYPE 2 DIABETES
One RCT (n = 101) showed a significantly greater reduction in A1C (−0.82% vs. −0.33%) at 10 weeks with FreeStyle Libre compared with SMBG, but no decrease in hypoglycemic events.14 A second RCT (n = 224) showed decreased time in hypoglycemia (−0.47 ± 0.13 hour per day) with FreeStyle Libre compared with SMBG, but no difference in A1C reduction, at six months.15 Neither RCT showed improvement in time in euglycemic range or hyperglycemic time or events.14,15
UTILIZATION CHANGES
Harms
Most device-related adverse effects are mild. The most common adverse effects are symptoms at the sensor site (itching, rash, pain, erythema, or bleeding), which can be treated without medical intervention or with topical medications.8–10,15 Other reported problems include technical issues, premature sensor loss, glucose measurement discrepancies, and visibility of sensor on the arm.10,13,16 About 12% to 15% of patients discontinued use of FreeStyle Libre, most commonly because of adverse effects or premature sensor loss.8,13
Cost
FreeStyle Libre costs approximately $75 for a reader and $135 per month for sensors without insurance.17,18 Medicare covers FreeStyle Libre for patients who are self-monitoring glucose four or more times a day, are being treated with insulin (three or more daily injections or infusion pump), and have required frequent insulin adjustments for a minimum of 60 days at the time of the prescription.17
In comparison, the initial cost of SMBG includes $20 for a monitor and $10 for a lancing device. Monthly costs include tests strips ($160 for 100 strips) and lancets ($12 for 100 lancets).18 With increasing test frequency, the cost of SMBG increases, but the cost of flash CGM stays the same.
Bottom Line
The use of FreeStyle Libre increases time in euglycemic range and decreases hyperglycemic and hypoglycemic time and events in adults with type 1 diabetes. There is limited evidence that it modestly reduces A1C levels in children with type 1 diabetes. In adults with insulin-treated type 2 diabetes, there is conflicting evidence whether it reduces A1C levels and hypoglycemic time and events.
The convenience, possible cost savings, and improvement in treatment satisfaction make FreeStyle Libre a good option for patients using insulin. However, SMBG should be used to confirm hypoglycemic values, during times of rapidly changing glucose levels (after meals and during exercise), and if symptoms do not correlate with flash CGM readings.
