
Am Fam Physician. 2021;104(1):16-19
Author disclosure: No relevant financial affiliations.
The efficient selection of drug therapy, timely dose titration, and avoidance of adverse events largely remain a trial and error process. For common diseases, such as hypertension and depression, several attempts are often required to find an effective drug, and optimizing the dose may take weeks to months. Patient adherence can decline during this process, which is compounded by adverse events that, although quantifiable in populations, seem to occur randomly in individual patients.
The enormous genetic variation underlying drug metabolism partially explains differing therapeutic responses and adverse events among patients. Differences in drug metabolism have driven pharmacogenetic testing in the clinic. Claims about pharmacogenetic testing, however, are inconsistently supported by scientific evidence, and most tests have not been examined by the U.S. Food and Drug Administration (FDA) or another objective regulatory body. Neither the Centers for Medicare and Medicaid Services nor the Centers for Disease Control and Prevention, under the Clinical Laboratory Improvement Amendments of 1998, evaluates tests or reviews their claims before entering the market.
In a safety communication to the public in 2018, the FDA expressed its concerns with pharmacogenetic tests whose claims have not been reviewed by the FDA.1 For example, it has not been established that the effectiveness of antidepressant medications is related to DNA variations and therefore is not a basis on which to select therapy. In the treatment of depression, relying on tests that are not supported by valid scientific evidence could lead to avoidance of first-line medications in favor of alternatives with less evidence of safety and effectiveness, prolonged trials of medications that do not alleviate symptoms, or reluctance to increase to higher dosages that could be effective.
To share its perspective on the state of the science, the FDA has published a table of pharmacogenetic associations related to genetic variants that can affect drug concentrations, therapeutic responses, or adverse events.2,3 The table catalogs gene-drug associations that the FDA has evaluated, finding sufficient scientific evidence to suggest that subgroups of patients are likely to have altered drug metabolism and, in certain cases, differential therapeutic effects and/or risks of adverse events. Select examples from the FDA table that may be of interest to family physicians are shown in Table 1.2
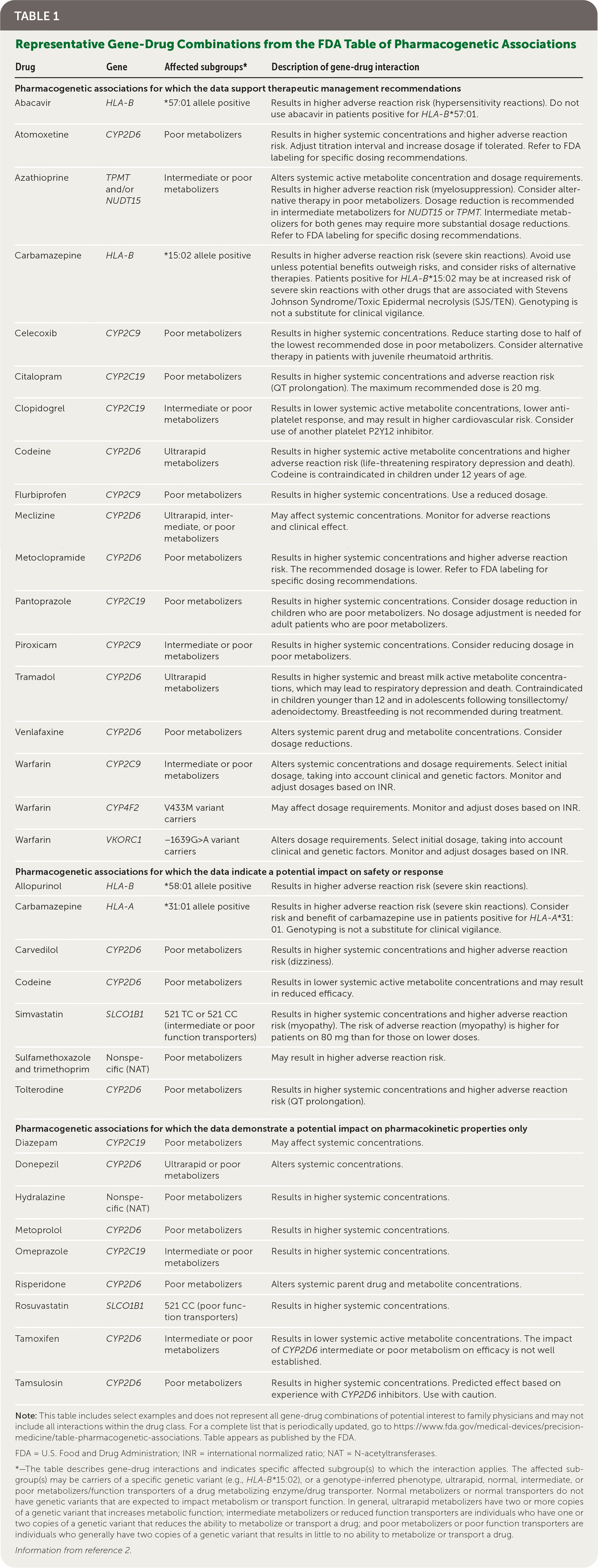
| Drug | Gene | Affected subgroups* | Description of gene-drug interaction |
|---|---|---|---|
| Pharmacogenetic associations for which the data support therapeutic management recommendations | |||
| Abacavir | HLA-B | *57:01 allele positive | Results in higher adverse reaction risk (hypersensitivity reactions). Do not use abacavir in patients positive for HLA-B*57:01. |
| Atomoxetine | CYP2D6 | Poor metabolizers | Results in higher systemic concentrations and higher adverse reaction risk. Adjust titration interval and increase dosage if tolerated. Refer to FDA labeling for specific dosing recommendations. |
| Azathioprine | TPMT and/or NUDT15 | Intermediate or poor metabolizers | Alters systemic active metabolite concentration and dosage requirements. Results in higher adverse reaction risk (myelosuppression). Consider alter- native therapy in poor metabolizers. Dosage reduction is recommended in intermediate metabolizers for NUDT15 or TPMT. Intermediate metabolizers for both genes may require more substantial dosage reductions. Refer to FDA labeling for specific dosing recommendations. |
| Carbamazepine | HLA-B | *15:02 allele positive | Results in higher adverse reaction risk (severe skin reactions). Avoid use unless potential benefits outweigh risks, and consider risks of alternative therapies. Patients positive for HLA-B*15:02 may be at increased risk of severe skin reactions with other drugs that are associated with Stevens Johnson Syndrome/Toxic Epidermal necrolysis (SJS/TEN). Genotyping is not a substitute for clinical vigilance. |
| Celecoxib | CYP2C9 | Poor metabolizers | Results in higher systemic concentrations. Reduce starting dose to half of the lowest recommended dose in poor metabolizers. Consider alternative therapy in patients with juvenile rheumatoid arthritis. |
| Citalopram | CYP2C19 | Poor metabolizers | Results in higher systemic concentrations and adverse reaction risk (QT prolongation). The maximum recommended dose is 20 mg. |
| Clopidogrel | CYP2C19 | Intermediate or poor metabolizers | Results in lower systemic active metabolite concentrations, lower anti- platelet response, and may result in higher cardiovascular risk. Consider use of another platelet P2Y12 inhibitor. |
| Codeine | CYP2D6 | Ultrarapid metabolizers | Results in higher systemic active metabolite concentrations and higher adverse reaction risk (life-threatening respiratory depression and death). Codeine is contraindicated in children under 12 years of age. |
| Flurbiprofen | CYP2C9 | Poor metabolizers | Results in higher systemic concentrations. Use a reduced dosage. |
| Meclizine | CYP2D6 | Ultrarapid, intermediate, or poor metabolizers | May affect systemic concentrations. Monitor for adverse reactions and clinical effect. |
| Metoclopramide | CYP2D6 | Poor metabolizers | Results in higher systemic concentrations and higher adverse reaction risk. The recommended dosage is lower. Refer to FDA labeling for specific dosing recommendations. |
| Pantoprazole | CYP2C19 | Poor metabolizers | Results in higher systemic concentrations. Consider dosage reduction in children who are poor metabolizers. No dosage adjustment is needed for adult patients who are poor metabolizers. |
| Piroxicam | CYP2C9 | Intermediate or poor metabolizers | Results in higher systemic concentrations. Consider reducing dosage in poor metabolizers. |
| Tramadol | CYP2D6 | Ultrarapid metabolizers | Results in higher systemic and breast milk active metabolite concentrations, which may lead to respiratory depression and death. Contraindicated in children younger than 12 and in adolescents following tonsillectomy/adenoidectomy. Breastfeeding is not recommended during treatment. |
| Venlafaxine | CYP2D6 | Poor metabolizers | Alters systemic parent drug and metabolite concentrations. Consider dosage reductions. |
| Warfarin | CYP2C9 | Intermediate or poor metabolizers | Alters systemic concentrations and dosage requirements. Select initial dosage, taking into account clinical and genetic factors. Monitor and adjust dosages based on INR. |
| Warfarin | CYP4F2 | V433M variant carriers | May affect dosage requirements. Monitor and adjust doses based on INR. |
| Warfarin | VKORC1 | –1639G>A variant carriers | Alters dosage requirements. Select initial dosage, taking into account clinical and genetic factors. Monitor and adjust dosages based on INR. |
| Pharmacogenetic associations for which the data indicate a potential impact on safety or response | |||
| Allopurinol | HLA-B | *58:01 allele positive | Results in higher adverse reaction risk (severe skin reactions). |
| Carbamazepine | HLA-A | *31:01 allele positive | Results in higher adverse reaction risk (severe skin reactions). Consider risk and benefit of carbamazepine use in patients positive for HLA-A*31: 01. Genotyping is not a substitute for clinical vigilance. |
| Carvedilol | CYP2D6 | Poor metabolizers | Results in higher systemic concentrations and higher adverse reaction risk (dizziness). |
| Codeine | CYP2D6 | Poor metabolizers | Results in lower systemic active metabolite concentrations and may result in reduced efficacy. |
| Simvastatin | SLCO1B1 | 521 TC or 521 CC (intermediate or poor function transporters) | Results in higher systemic concentrations and higher adverse reaction risk (myopathy). The risk of adverse reaction (myopathy) is higher for patients on 80 mg than for those on lower doses. |
| Sulfamethoxazole and trimethoprim | Nonspecific (NAT) | Poor metabolizers | May result in higher adverse reaction risk. |
| Tolterodine | CYP2D6 | Poor metabolizers | Results in higher systemic concentrations and higher adverse reaction risk (QT prolongation). |
| Pharmacogenetic associations for which the data demonstrate a potential impact on pharmacokinetic properties only | |||
| Diazepam | CYP2C19 | Poor metabolizers | May affect systemic concentrations. |
| Donepezil | CYP2D6 | Ultrarapid or poor metabolizers | Alters systemic concentrations. |
| Hydralazine | Nonspecific (NAT) | Poor metabolizers | Results in higher systemic concentrations. |
| Metoprolol | CYP2D6 | Poor metabolizers | Results in higher systemic concentrations. |
| Omeprazole | CYP2C19 | Intermediate or poor metabolizers | Results in higher systemic concentrations. |
| Risperidone | CYP2D6 | Poor metabolizers | Alters systemic parent drug and metabolite concentrations. |
| Rosuvastatin | SLCO1B1 | 521 CC (poor function transporters) | Results in higher systemic concentrations. |
| Tamoxifen | CYP2D6 | Intermediate or poor metabolizers | Results in lower systemic active metabolite concentrations. The impact of CYP2D6 intermediate or poor metabolism on efficacy is not well established. |
| Tamsulosin | CYP2D6 | Poor metabolizers | Results in higher systemic concentrations. Predicted effect based on experience with CYP2D6 inhibitors. Use with caution. |
The gene-drug interactions listed in the table include those described in FDA-approved drug labeling and may also reflect associations that are well documented in the scientific literature. Inclusion of gene-drug interactions in the table does not imply that the FDA advocates genetic testing for every drug listed; however, for certain drugs, such as abacavir (Ziagen), eliglustat (Cerdelga), and siponimod (Mayzent), genetic testing is essential for their safe and effective use; specific prescribing recommendations are noted where available. Clinicians can use this table to supplement other evidence sources and patient-specific factors when making prescribing decisions and should refer to FDA-approved labeling for complete prescribing information.
In certain cases, scientific evidence supports a relationship between specific genes with well-characterized variations and their effects on the metabolism of particular drugs, influencing the rate of adverse events and/or supporting different dosing considerations. In such cases, pharmacogenetic testing may have value. One example is CYP2C19 intermediate or poor metabolizers, in whom clopidogrel (Plavix) may result in lower antiplatelet response and higher cardiovascular risk. In such patients, prescribers should consider use of another platelet P2Y12 inhibitor. Another example is carriers of the HLA-B*57:01 allele, who should not take abacavir because they are at higher risk of hypersensitivity reactions to the drug.
In many other cases, gene-drug interactions have a potential impact on drug safety or response, but the evidence is insufficient to support a specific clinical action. The FDA table also lists gene-drug interactions that affect pharmacokinetic properties only (e.g., ultrarapid or poor metabolizers; poor function transporters), but for which the impact on safety or response of the corresponding drug has not been established.
The FDA's table of pharmacogenetic associations is currently limited to the scope discussed in this editorial; various other pharmacogenetic associations exist but are not yet listed. The table will be updated periodically with additional pharmacogenetic associations supported by sufficient scientific evidence. The FDA has opened a docket for public comment4 and encourages stakeholders to provide feedback on specific associations that should or should not be included in future updates. Further, the FDA encourages collaborative efforts and is currently a member of the Standardizing Laboratory Practices in Pharmacogenomics (STRIPE) Collaborative Community.5,6 The FDA will continue to communicate about the practical uses of pharmacogenetic testing, as well as about concerns that may impact public health.
