
Am Fam Physician. 2021;104(1):26-27
Author disclosure: No relevant financial affiliations.

Details for This Review
Study Population: Patients 22 to 75 years of age with histologic evidence of endometrial hyperplasia
Efficacy End Points: Regression of endometrial hyperplasia when treated with the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) for six months or less and for 12 months (regression was defined as biopsy showing return to normal or a change from atypical hyperplasia to endometrial hyperplasia without atypia); avoidance of hysterectomy for a malignant or nonmalignant reason
Harm End Points: Requested removal of the LNG-IUS because of adverse effects (e.g., spotting, nausea, weight gain)
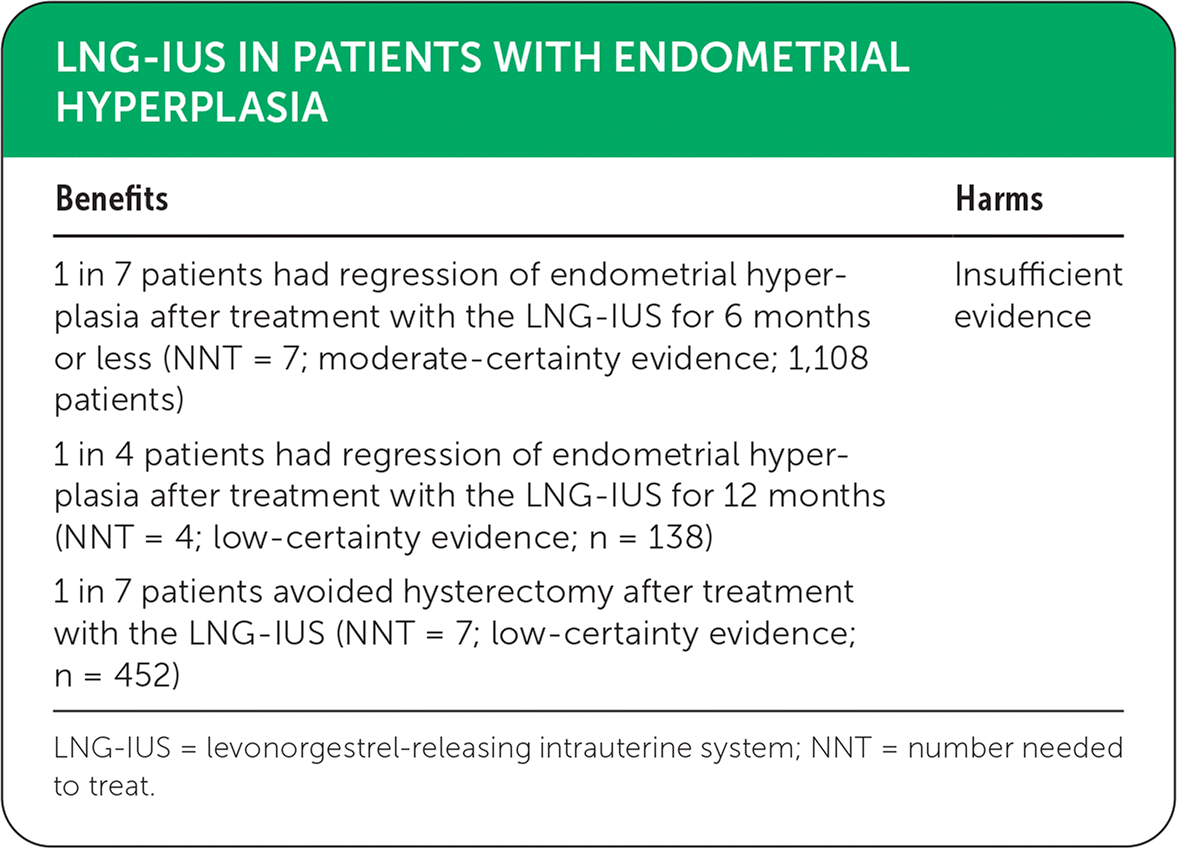
| Benefits | Harms |
|---|---|
| 1 in 7 patients had regression of endometrial hyperplasia after treatment with the LNG-IUS for 6 months or less (NNT = 7; moderate-certainty evidence; 1,108 patients) | Insufficient evidence |
| 1 in 4 patients had regression of endometrial hyperplasia after treatment with the LNG-IUS for 12 months (NNT = 4; low-certainty evidence; n = 138) | |
| 1 in 7 patients avoided hysterectomy after treatment with the LNG-IUS (NNT = 7; low-certainty evidence; n = 452) |
Narrative: Endometrial hyperplasia is the excessive production of cells in the endometrium caused by unopposed estrogen states, such as obesity, anovulatory bleeding, and hormone therapy. Endometrial hyperplasia is the precursor to endometrial cancer, the sixth most common cancer worldwide.1 Progesterone therapy leads to regression of endometrial hyperplasia and prevention of endometrial cancer. The most common regimen is six months of intramuscular or high-dose oral progesterone, either of which can cause significant adverse effects. Surgery is another option, but its use may be limited for those wishing to maintain fertility.1
This Cochrane review of 13 randomized controlled trials (RCTs) included 3,174 patients from Egypt, Iran, China, Turkey, Kuwait, Pakistan, and Norway. Treatment of endometrial hyperplasia with the LNG-IUS (1,657 patients) was compared with systemic progesterone (1,327 patients) or no treatment (190 patients).1 Systemic progesterone was administered orally in 12 studies and intramuscularly in one study. Endometrial hyperplasia was diagnosed through endometrial sampling (endometrial biopsy or suction curettage) with histologic evaluation. Twelve trials included endometrial hyperplasia without atypia, and one study included endometrial hyperplasia with atypia. Sampling with histologic examination was performed again at the end of the treatment period to determine treatment effectiveness.
Treatment with the LNG-IUS ranged from three months to 24 months in 11 RCTs. Two of the RCTs did not have enough information about treatment duration to include in analysis. Overall, by the end of the treatment period, the LNG-IUS improved regression of endometrial hyperplasia compared with systemic progesterone or no treatment (at six months or less of treatment, odds ratio [OR] = 2.94; 95% CI, 2.10 to 4.13; n = 1,108; at 12 months of treatment, OR = 3.80; 95% CI, 1.75 to 8.23; n = 138).1 At six months or less of treatment, the rate of regression was 86% with the LNG-IUS and 72% with systemic progesterone. At 12 months, these rates were 80% with the LNG-IUS and 51% with systemic progesterone.
Although the number of patients was small and the indications for hysterectomy were poorly defined, treatment with the LNG-IUS was more effective overall at preventing hysterectomy than oral progesterone. In those treated with the LNG-IUS, the rate of hysterectomy was 11%, compared with 26% in those treated with oral progesterone (OR = 0.26; 95% CI, 0.15 to 0.46; n = 452).1
There was insufficient evidence to determine device-related effects (e.g., expulsion). The most common general adverse effects reported with both the LNG-IUS and systemic progesterone were spotting, nausea, and weight gain. Four of the RCTs examined adverse effects requiring treatment discontinuation. There was no statistical difference between the groups in the rate of withdrawal because of adverse effects.1
Caveats: This meta-analysis showed overall moderate-certainty evidence for using the LNG-IUS for regression of endometrial hyperplasia vs. systemic progesterone or no treatment. All but one of the studies included only those with endometrial hyperplasia without atypia. Therefore, the use of the LNG-IUS in patients who have endometrial hyperplasia with atypia requires more research. The treatment dosages for the LNG-IUS were reported in nine out of the 13 studies, with most using 20 mcg per day (n = 8).1 Based on this, a dosage of 20 mcg per day is reasonable for treating endometrial hyperplasia, but more research could help delineate the optimal dosage.
The evidence for using the LNG-IUS for prevention of hysterectomy was of low certainty. The reason for hysterectomy was not clear in most studies and included malignant reasons (e.g., endometrial cancer) and nonmalignant reasons (e.g., menorrhagia). There is longstanding high-certainty evidence for the many established benefits of the LNG-IUS, including decreased menorrhagia and fibroid volume regression, which, if untreated, could otherwise become nonmalignant reasons for hysterectomy.2
Finally, only eight studies reported the menopausal status of participants, with 731 being premenopausal and 193 being postmenopausal.1 The LNG-IUS is an alternative to hysterectomy in management of endometrial intraepithelial neoplasia and endometrial hyperplasia for patients who wish to maintain fertility.3 This may account for why there were more premenopausal patients in the studies.
Conclusion: The American College of Obstetricians and Gynecologists recognizes that the LNG-IUS for treatment of endometrial hyperplasia is an alternative to hysterectomy in appropriate patients.3 This Cochrane review supports a rating of green for this treatment option, showing successful regression in one out of seven patients at six months of treatment and one out of three patients at 12 months of treatment. Furthermore, most patients tolerated treatment with the LNG-IUS with only minimal adverse effects, and only one out of 41 requested treatment withdrawal. Further research should be targeted at optimal LNG-IUS treatment dosing and duration.
The views expressed within this publication represent those of the authors and do not reflect the official position of U.S. Air Force, Uniformed Services University of the Health Sciences, the U.S. government, or the Department of Defense at large.
