
Am Fam Physician. 2021;104(1):93-94
Author disclosure: No relevant financial affiliations.
Clascoterone (Winlevi) is an androgen receptor inhibitor labeled for the topical treatment of acne vulgaris in patients 12 years and older.
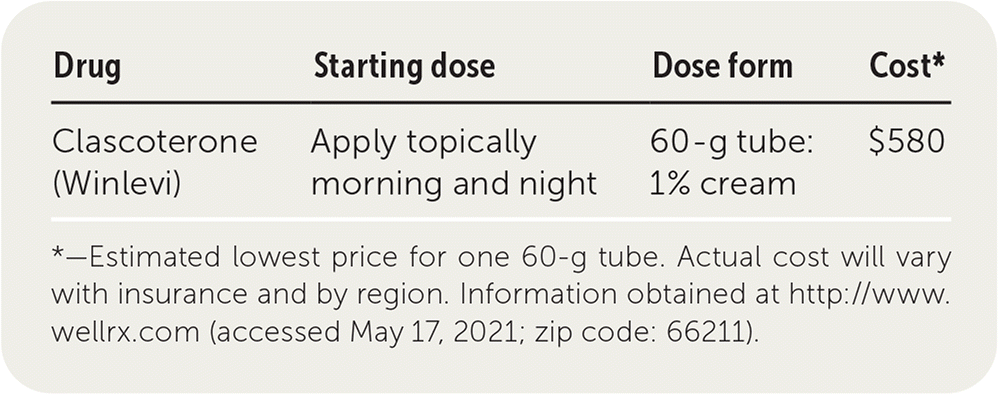
| Drug | Starting dose | Dose form | Cost* |
|---|---|---|---|
| Clascoterone (Winlevi) | Apply topically morning and night | 60-g tube: 1% cream | $580 |
Safety
Two studies of 722 patients receiving clascoterone demonstrated a low frequency of adverse effects related to treatment.1,2 The overall safety profile of clascoterone in these studies was similar to that of the vehicle cream.3,4 Topical use of clascoterone has low systemic exposure.1 However, given the structural similarities to the oral antiandrogen spironolactone, which can cause systemic adverse effects such as hyperkalemia, gynecomastia, and menstrual irregularities, additional study was required.5 Laboratory-detected hypothalamic-pituitary-adrenal axis suppression and hyperkalemia occurred in a small number of patients with no significant clinical impact on reported symptoms or blood pressure.1 Clascoterone is not labeled for use in children younger than 12 years. There are limited data about the safety of clascoterone during pregnancy, and further research is needed on the effects of clascoterone, including the risk of major birth defects, miscarriage, and adverse maternal or fetal outcomes.1,2
Tolerability
Effectiveness
Two randomized controlled studies that included a total of 1,440 patients with moderate to severe acne demonstrated an increased treatment success rate and a total reduction in the number of inflammatory and noninflammatory lesions after 12 weeks of treatment with clascoterone compared with vehicle cream.3 Moderate to severe acne was defined as a score of 3 or 4 on a 5-point Investigator's Global Assessment scale. Treatment success was defined as an assessment score of 0 (clear), 1 (almost clear), or at least a 2-point improvement from baseline and an overall reduction in the number of lesions. After 12 weeks of treatment, the likelihood of treatment success was greater in both studies (18.4% vs. 9.0% with vehicle in study 1 and 20.3% vs. 6.5% with vehicle in study 2 [number needed to treat = 7 to 11]).3 However, with the exception of isotretinoin therapy, moderate to severe acne is usually treated with two or more medications to produce higher success rates. Clascoterone has not been evaluated in combination with other treatments.
Price
One 60-g tube of 1% clascoterone cream costs about $580. It is more expensive than other topical prescription and over-the-counter treatment options. In comparison, a 30-day supply of the generic oral antiandrogen spironolactone costs about $10.
Simplicity
Clascoterone is applied to clean, dry skin in the morning and evening. The use of other abrasive or topical agents, such as soaps or cosmetics, can intensify irritation and should be avoided if it occurs.2
Bottom Line
More comparative data are needed to justify the use of clascoterone over less expensive, currently available therapies for moderate to severe acne. However, systemic absorption of clascoterone is low, and it may serve as a safe alternative for patients 12 years and older who saw improvements from oral antiandrogen therapy but could not tolerate systemic adverse effects such as blood pressure changes or gynecomastia.
