
Am Fam Physician. 2021;104(3):online
Author disclosure: No relevant financial affiliations.

Details for This Review
Study Population: 68 randomized controlled trials (RCTs) comprising 22,975 patients with Helicobacter pylori infection
Efficacy End Points: Cure rates
Harm End Points: Not assessed
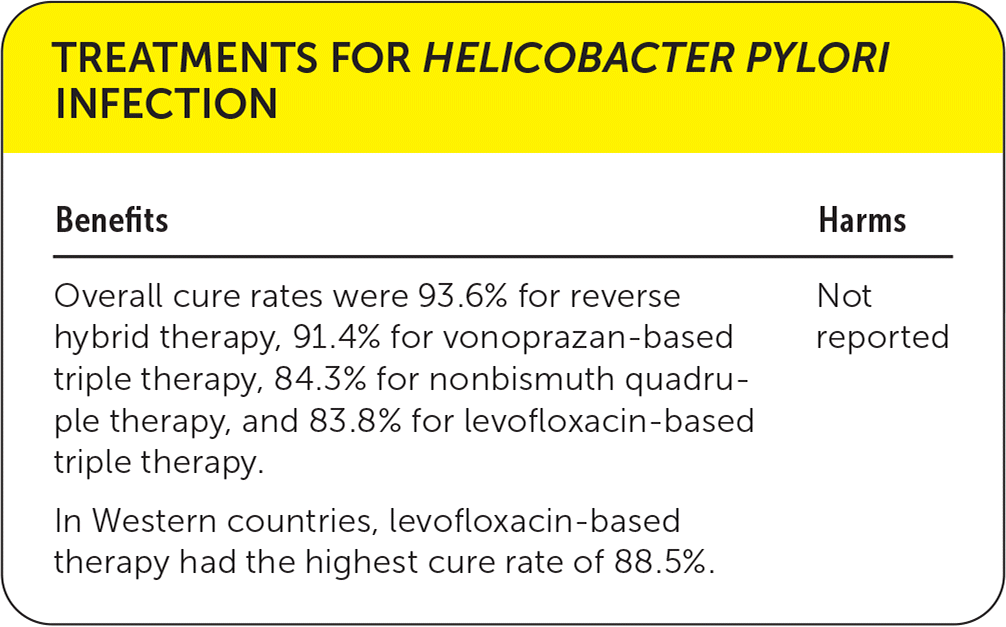
| Benefits | Harms |
|---|---|
| Overall cure rates were 93.6% for reverse hybrid therapy, 91.4% for vonoprazan-based triple therapy, 84.3% for nonbismuth quadruple therapy, and 83.8% for levofloxacin-based triple therapy. | Not reported |
| In Western countries, levofloxacin-based therapy had the highest cure rate of 88.5%. |
Narrative: H. pylori is a common infection associated with gastritis, peptic ulcers, gastric cancer, and mucosa-associated lymphoid tissue lymphoma.1 Current guidelines recommend treatment for all individuals with H. pylori infection to reduce complications.2,3 Despite a range of therapy options and regimens, cure rates are lower than treatments for other infectious diseases.1,4–6 A potassium-competitive acid blocker, vonoprazan (Takecab), was approved for use in Japan in 2015 to improve cure rates in patients with H. pylori infection, but it is not approved for use in the United States.7
This network meta-analysis included RCTs evaluating the effectiveness of empiric first-line dual, triple, and quadruple treatment regimens of seven days or longer for H. pylori infection.6 A network meta-analysis allows for direct and indirect comparison of treatment arms from different trials.8,9 The primary outcome of this meta-analysis was infection cure rate, and the authors subsequently ranked the overall effectiveness of each regimen. The authors also used surface under the cumulative ranking curve (SUCRA) values in intervention network charts to evaluate cumulative ranking probability for each intervention.9 The SUCRA value is used to evaluate which treatment is the most effective, ranging from 0% to 100%. Treatments with higher SUCRA values are considered more effective, and treatments with lower SUCRA values are considered less effective.9
The meta-analysis included 68 RCTs and 22,975 individuals. There were 56 two-arm and 12 three-arm RCTs, including 92 paired comparisons, which the authors grouped into 12 pairwise meta-analyses.6 A total of eight first-line treatment regimens allowed for 28 possible comparisons, of which 12 were direct and 16 were indirect (Table 1).6
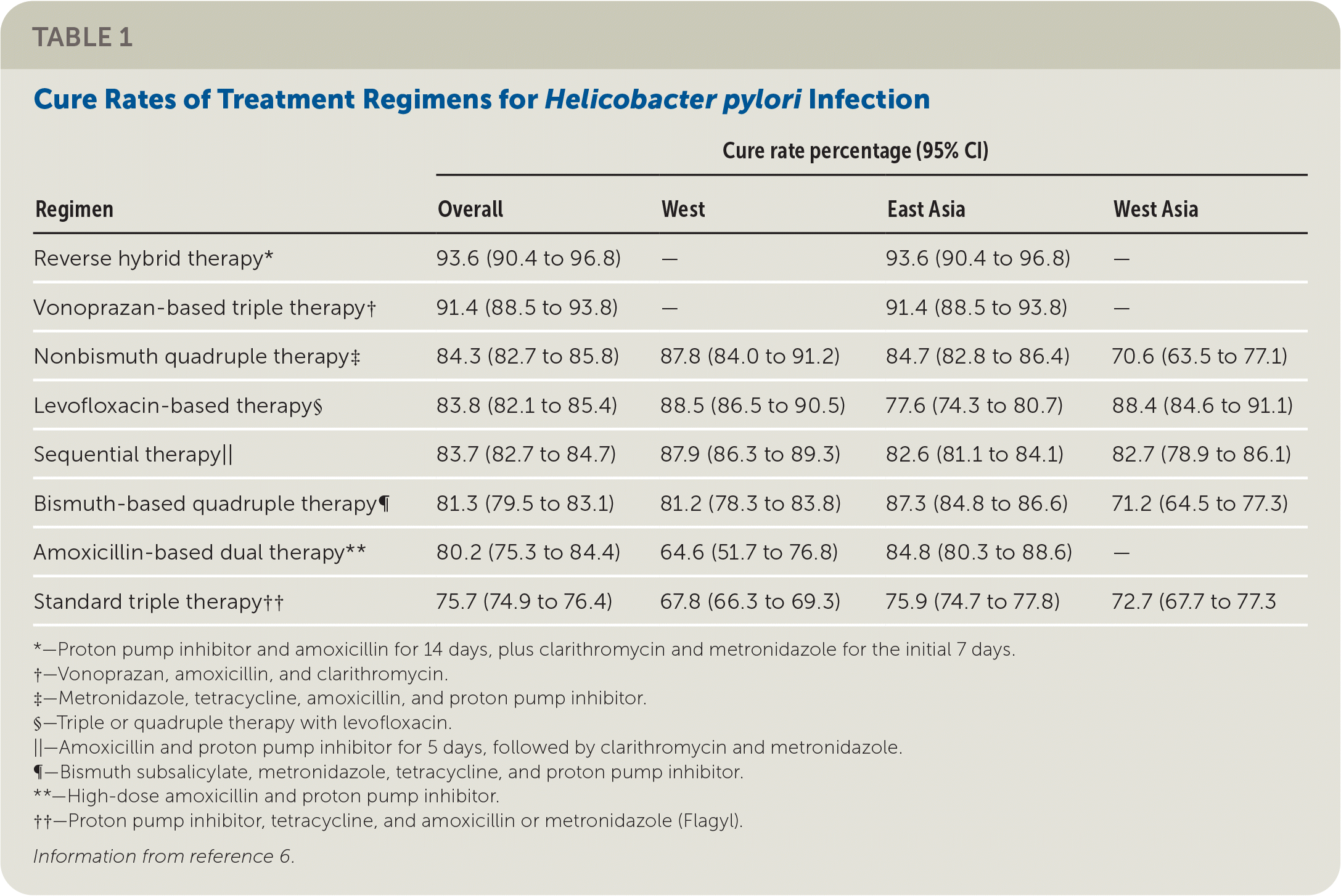
| Regimen | Cure rate percentage (95% CI) | |||
|---|---|---|---|---|
| Overall | West | East Asia | West Asia | |
| Reverse hybrid therapy* | 93.6 (90.4 to 96.8) | — | 93.6 (90.4 to 96.8) | — |
| Vonoprazan-based triple therapy† | 91.4 (88.5 to 93.8) | — | 91.4 (88.5 to 93.8) | — |
| Nonbismuth quadruple therapy‡ | 84.3 (82.7 to 85.8) | 87.8 (84.0 to 91.2) | 84.7 (82.8 to 86.4) | 70.6 (63.5 to 77.1) |
| Levofloxacin-based therapy§ | 83.8 (82.1 to 85.4) | 88.5 (86.5 to 90.5) | 77.6 (74.3 to 80.7) | 88.4 (84.6 to 91.1) |
| Sequential therapy|| | 83.7 (82.7 to 84.7) | 87.9 (86.3 to 89.3) | 82.6 (81.1 to 84.1) | 82.7 (78.9 to 86.1) |
| Bismuth-based quadruple therapy¶ | 81.3 (79.5 to 83.1) | 81.2 (78.3 to 83.8) | 87.3 (84.8 to 86.6) | 71.2 (64.5 to 77.3) |
| Amoxicillin-based dual therapy** | 80.2 (75.3 to 84.4) | 64.6 (51.7 to 76.8) | 84.8 (80.3 to 88.6) | — |
| Standard triple therapy†† | 75.7 (74.9 to 76.4) | 67.8 (66.3 to 69.3) | 75.9 (74.7 to 77.8) | 72.7 (67.7 to 77.3 |
Vonoprazan-based triple therapy and reverse hybrid therapy demonstrated the highest overall cure rates and SUCRA values; however, among Western countries, levofloxacin-based triple therapy had the highest cure rate and SUCRA value. Standard triple therapy (this study used a proton pump inhibitor, tetracycline, and amoxicillin or metronidazole [Flagyl]) was the least effective regimen overall.
All pairwise comparisons significantly supported the newer intervention over standard triple therapy: vonoprazan-based triple therapy vs. triple therapy (odds ratio [OR] = 3.80; 95% CI, 1.62 to 8.94), sequential therapy vs. triple therapy (OR = 1.79; 95% CI, 1.26 to 2.53), nonbismuth quadruple therapy vs. triple therapy (OR = 2.08; 95% CI, 1.45 to 2.98), bismuth-based quadruple therapy vs. triple therapy (OR = 1.47; 95% CI, 1.02 to 2.11), and levofloxacin-based therapy vs. triple therapy (OR = 1.79; 95% CI, 1.26 to 2.53).
Caveats: Although network meta-analyses are powerful tools for the comparative effectiveness of multiple treatment modalities when there are not enough direct pairwise comparison trials available, they are generally more complex and require more stringent planning and methodological rigor than pairwise meta-analyses. The assumptions of transitivity (i.e., if the relationship between a first and second element holds between the first and third element) and consistency are essential to the validity of the results.10
There are several limitations associated with this network meta-analysis. Most of the included RCTs are open-label and not double-blind, resulting in a lack of allocation concealment and blinding to the treatment arms. Despite this, most studies used objective outcome measurement and blinded staff who were performing the test to the treatment groups. Another limitation is that resistance was not considered in most included studies, which does not allow for subgroup analysis based on resistance. Treatment safety was not assessed in most studies, and authors did not report data on harms. Of the 68 RCTs included in the meta-analysis, vonoprazan-based triple therapy was only tested in three trials with 897 patients from Japan, and reverse hybrid therapy was assessed in only one trial with 440 patients and was not found to be significantly better than triple therapy. Further randomized controlled trials are needed to evaluate vonoprazan-based triple therapy, including in Western and Western Asian populations.
Conclusion: Based on existing evidence, we have assigned a color recommendation of yellow (data inadequate) for the use of vonoprazan-based triple therapy or reverse hybrid therapy in East Asia and levofloxacin-based triple therapy in Western countries. Further data from double-blind RCTs in other populations and data on the risk of adverse events are needed.
