
Am Fam Physician. 2021;104(3):311-312
Author disclosure: No relevant financial affiliations.
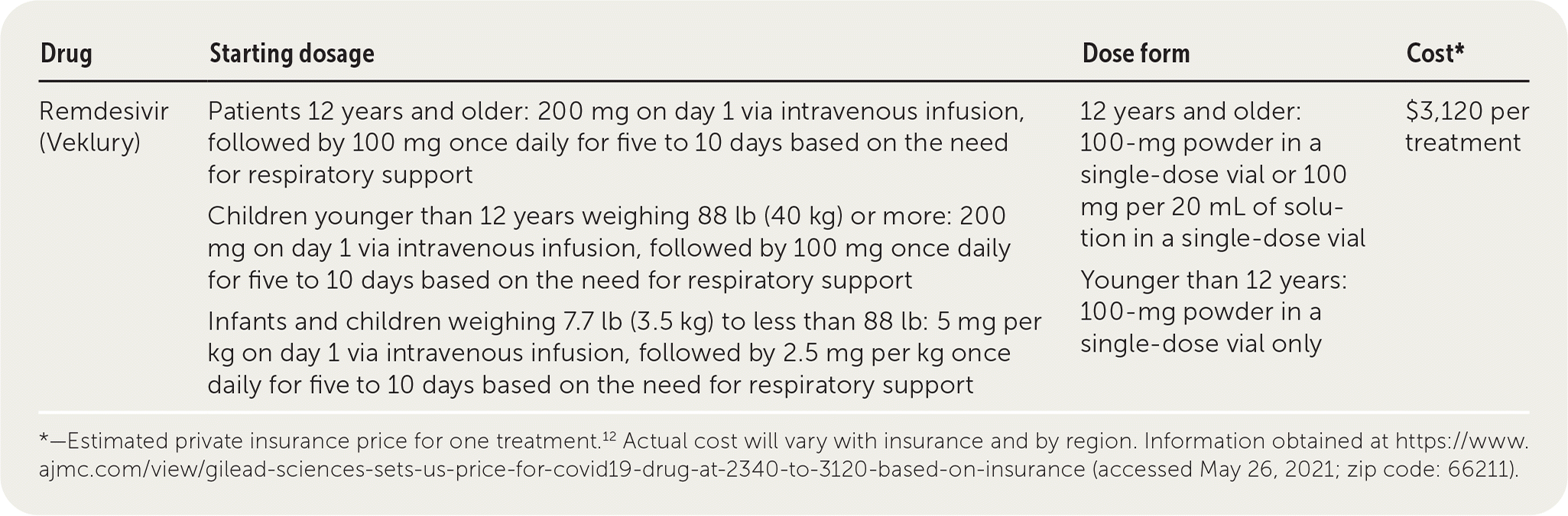
Remdesivir (Veklury) is an antiviral drug that inhibits the replication of pathogenic human coronaviruses, including SARS-CoV-2 and SARS-CoV-1.1 Remdesivir is approved by the U.S. Food and Drug Administration for the treatment of COVID-19 in adults, and it can be given to children 12 years and older (weighing at least 88 lb [40 kg]) or younger than 12 years (weighing at least 7.7 lb [3.5 kg]) under an emergency use authorization.2
| Drug | Starting dosage | Dose form | Cost* |
|---|---|---|---|
| Remdesivir (Veklury) | Patients 12 years and older: 200 mg on day 1 via intravenous infusion, followed by 100 mg once daily for five to 10 days based on the need for respiratory support Children younger than 12 years weighing 88 lb (40 kg) or more: 200 mg on day 1 via intravenous infusion, followed by 100 mg once daily for five to 10 days based on the need for respiratory support Infants and children weighing 7.7 lb (3.5 kg) to less than 88 lb: 5 mg per kg on day 1 via intravenous infusion, followed by 2.5 mg per kg once daily for five to 10 days based on the need for respiratory support | 12 years and older: 100-mg powder in a single-dose vial or 100 mg per 20 mL of solution in a single-dose vial Younger than 12 years: 100-mg powder in a single-dose vial only | $3,120 per treatment |
Safety
Based on data from three phase 3 studies that included 1,313 patients, the most common adverse effects leading to discontinuation of treatment are infusion-related hypersensitivity reactions, which occur in less than 1% of patients.2,3 Remdesivir should not be used in patients with an estimated glomerular filtration rate (GFR) of less than 30 mL per minute and should be discontinued if the estimated GFR decreases to less than 30 mL per minute. Because remdesivir may affect liver function, patients should be monitored. If alanine transaminase levels increase to more than 10 times the upper limit of normal, treatment must be discontinued. Remdesivir should also be discontinued if the patient exhibits signs and symptoms of liver inflammation, acute kidney injury with decreased GFR, and decreased heart rate.3–5 There are no available data on the drug-associated risk of major birth defects or the effects on breastfed infants in patients who are pregnant or breastfeeding.2 Remdesivir should not be used concurrently with chloroquine phosphate or hydroxychloroquine sulfate because antiviral activity may be reduced.2
Tolerability
Remdesivir is well tolerated by most adults with mild to moderate and severe COVID-19. Approximately 5% (10-day dosing regimen) and 3% (five-day dosing regimen) of patients discontinued treatment because of adverse effects.4 The tolerability of remdesivir in children younger than 12 years has not been established.
Effectiveness
Remdesivir has been evaluated in several studies in hospitalized patients with mild to moderate or severe COVID-19 symptoms. Benefit varies based on illness severity and outcome measured. In the ACTT-1 trial, which involved 1,062 patients with all levels of illness severity, remdesivir did not reduce time to recovery vs. placebo (five days vs. six days) among 119 patients with mild to moderate disease not requiring oxygen supplementation. However, it reduced the time to recovery from 20 days to 15 days among 193 patients requiring noninvasive ventilation or use of high-flow oxygen devices. Remdesivir did not benefit patients receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO).3 In the Solidarity trial (N = 11,330 patients), remdesivir therapy did not significantly reduce the in-hospital mortality rate to a greater degree than placebo among 3,204 patients not requiring oxygen supplementation or mechanical ventilation.6 In the GS-U.S.-540-5774 trial (N = 596 patients), remdesivir did not lead to a statistically significant improvement in clinical status compared with the standard of care at 11 days after initiation of treatment among 411 patients with moderate COVID-19 not requiring oxygen supplementation.7 In another trial that included 237 patients with severe COVID-19 requiring normal and high-flow oxygen supplementation, remdesivir did not provide any statistically significant clinical benefits compared with placebo.1
In addition, clinical practice guidelines differ in their recommendations as to whether remdesivir should be used. In patients with mild symptoms who require no, minimal, or increasing amounts of supplemental oxygen, the National Institutes of Health (NIH) COVID-19 treatment guidelines recommend using remdesivir, whereas the Infectious Diseases Society of America (IDSA) recommends its use only in patients requiring oxygen.5,8 For patients requiring invasive mechanical ventilation or ECMO, the NIH does not recommend starting treatment but suggests continuing treatment if respiratory support has been initiated.8 In contrast, the IDSA suggests using remdesivir in hospitalized patients who require mechanical ventilation or ECMO.9 The World Health Organization guideline for the clinical management of COVID-19 recommends against the use of remdesivir regardless of the level of need for oxygen supplementation, based on a systematic review and network meta-analysis that pooled data from four randomized trials of 7,333 participants hospitalized for COVID-19.1,3,6,7,9,10
Price
Simplicity
Remdesivir is administered as a single loading intravenous infusion of 200 mg, followed by a maintenance dosage of 100 mg per day for 10 days in patients requiring invasive mechanical ventilation or ECMO, or five days for patients who do not require ventilatory support.2 Creatinine levels, liver function tests, and prothrombin time should be determined in patients before starting treatment with remdesivir, and monitoring should be continued as clinically appropriate.2
Bottom Line
Although remdesivir has been shown to be safe and well tolerated, there is a lack of consistency in clinical trial results and guideline recommendations. The current evidence suggests that remdesivir can improve the time to recovery in patients hospitalized with COVID-19 who require supplemental oxygen, including noninvasive ventilation and the use of high-flow oxygen devices.
