
Am Fam Physician. 2022;105(2):207-209
Author disclosure: No relevant financial relationships.
Key Points for Practice
• HCM is evaluated and monitored with regular ECG, echocardiography, and prolonged cardiac monitoring.
• ECG results are abnormal in up to 95% of patients with HCM.
• Implantable cardiac defibrillators can reduce sudden cardiac death in patients with risk factors.
• Moderate intensity exercise is safe for patients with stable HCM.
From the AFP Editors
Hypertrophic cardiomyopathy (HCM) is a heritable, autosomal dominant disorder of structural abnormalities confined to the heart. The prevalence of asymptomatic HCM is between one in 500 and one in 200 U.S. adults, whereas an undefined fraction has symptomatic disease. Among patients with HCM, risk of sudden cardiac death is highest at a younger age. HCM is the most common cause of sudden cardiac death in young people; it occurs in up to 10% of patients after diagnosis in childhood. HCM leads to left ventricular hypertrophy, especially septal hypertrophy, which causes obstruction of the outflow from the left ventricle and distorts the mitral valve. These guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) update the previous guidance released by these organizations in 2011.
Evaluation of Suspected HCM
Workup for HCM should be considered in children and adults with a family history of HCM, and patients with heart murmur or abnormal electrocardiography (ECG) results. Initial workup includes a three-generation family history, dynamic cardiac examination, ECG, echocardiography, and prolonged cardiac monitoring. HCM can also present with symptoms associated with exertion, including chest pain, dyspnea, palpitations, or syncope. HCM is often incidentally detected after finding a heart murmur or abnormal results on ECG.
Genetic variants are found in only 30% to 60% of patients with HCM. Because eight genes have been associated with HCM, often with multiple pathologic variants, pretest genetic counseling by a counselor versed in cardiac genetics is highly recommended. If no genetic variants are found, periodic reevaluation should be considered as new variants continue to be discovered and their significance further refined.
Evaluation is aimed at detecting the abnormalities associated with HCM including dynamic left ventricular outflow tract obstruction (LVOTO), mitral regurgitation, diastolic dysfunction, myocardial ischemia, arrhythmias, and autonomic dysfunction. LVOTO is present in three-fourths of patients with HCM. Because LVOTO worsens with increased contractility, decreased preload, and decreased afterload, it is more likely to be detected with exertion or provocative maneuvers. In a dynamic cardiac examination, Valsalva or squat-to-stand maneuvers are used to increase LVOTO and the likelihood of detecting a murmur. ECG is essential because up to 95% of patients with HCM will have abnormalities on a 12-lead electrocardiogram. Prolonged cardiac monitoring is recommended because more than one-half of patients with HCM have paroxysmal atrial fibrillation.
Imaging with echocardiography or cardiovascular magnetic resonance imaging is the key to diagnosing HCM. In adults, HCM is diagnosed when maximal left ventricular end-diastolic wall thickness exceeds 15 mm at any point. When patients have a known associated gene variant or first-degree family member with HCM, wall thickness of 13 mm is diagnostic. Left ventricular wall thickness greater than two standard deviations above the mean for age is used in children to account for growth.
Differential Diagnosis
Several other common cardiac conditions also present with left ventricular hypertrophy, especially hypertensive cardiomyopathy and secondary remodeling from athletic activity. Aortic valvular abnormalities can also lead to left ventricular hypertrophy. Systemic and metabolic diseases such as amyloidosis, RASopathies (e.g., type 1 neurofibromatosis), glycogen and lysosomal storage diseases, as well as fat metabolism disorders can be associated with left ventricular hypertrophy that may mimic HCM. Dysmorphic features, metabolic derangements, and neurologic deficits should prompt genetic screening for these diseases.
Management
All patients with HCM should be managed in conjunction with a center specializing in HCM; such centers have better outcomes. When patients are asymptomatic, management is limited to routine monitoring of symptoms and imaging. In children, repeat ECG, echocardiography, and continuous cardiac monitoring should be completed every one to two years. In adults, this monitoring is recommended every three to five years. All first-degree family members of patients with HCM require the same assessment. Those whose relatives were diagnosed in childhood are evaluated every one to three years and those diagnosed as adults every three to five years.
In symptomatic patients, beta blockade is used to reduce the resting heart rate and LVOTO to treat symptoms. Beta-blocker doses must balance control of cardiac symptoms and symptomatic bradycardia. Nondihydropyridine calcium channel blockers are an alternative for those with significant adverse effects or insufficient control on beta blockers. The two agents should not be combined. If echocardiography does not show obstruction, loop or thiazide diuretics can be used to improve dyspnea if volume overload is present. However, diuretics, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers can increase LVOTO by vasodilation and decreased afterload. When echocardiography does not show obstruction but ejection fraction is less than 50%, it should be treated as heart failure with reduced ejection fraction. Hypotension should be avoided in HCM, and acute hypotension can lead to a medical emergency.
Patients who continue to have symptoms despite beta blockers or calcium channel blockers are candidates for disopyramide (Norpace), an antidysrhythmic agent, or septal reduction therapy. Disopyramide must be combined with an atrioventricular nodal blocking medication because it can lead to atrial fibrillation when used alone. Septal reduction by myectomy or alcohol ablation improves symptoms but does not prevent disease progression.
Implantable cardiac defibrillators reduce sudden cardiac death in those at increased risk (Table 1). Younger patients have higher complication risks from implantable cardiac defibrillator placement but receive the most benefit. Sudden cardiac death risk should be reevaluated during regular follow-up. Shared decision-making is paramount.
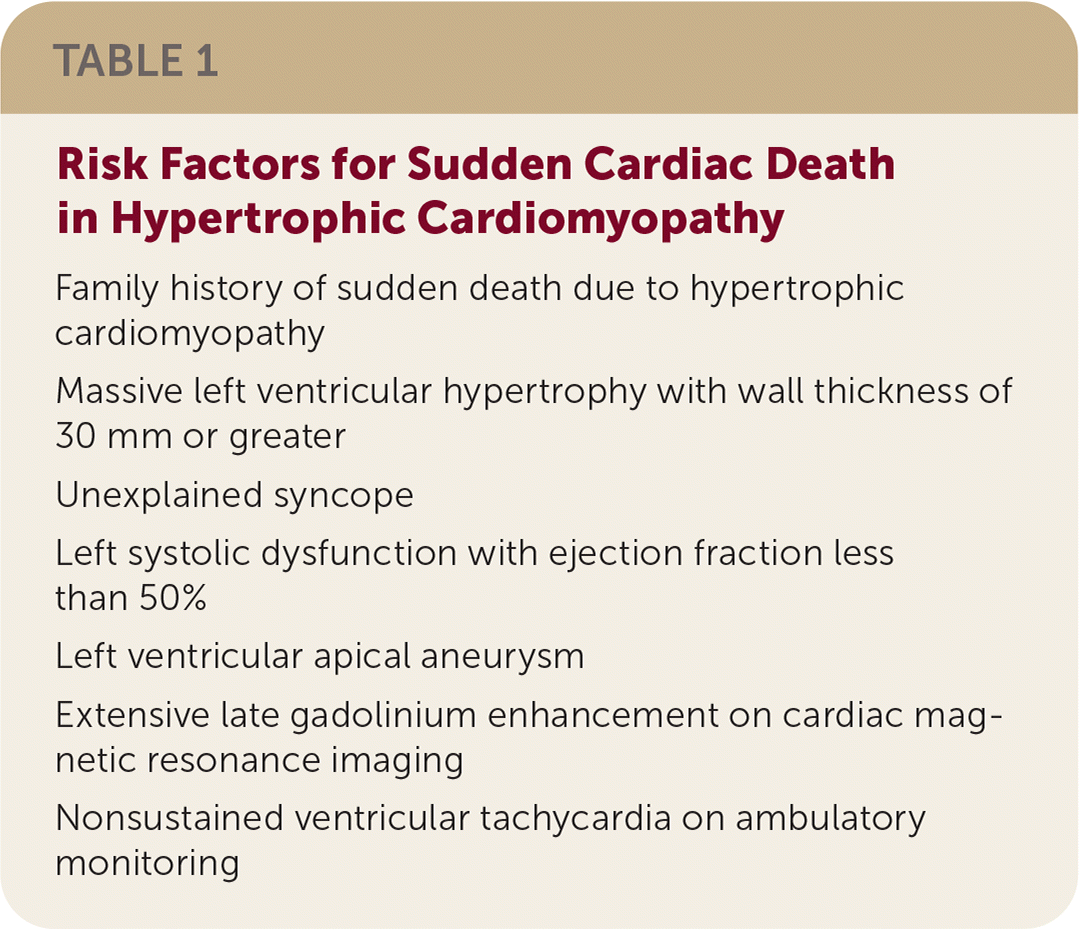
| Family history of sudden death due to hypertrophic cardiomyopathy |
| Massive left ventricular hypertrophy with wall thickness of 30 mm or greater |
| Unexplained syncope |
| Left systolic dysfunction with ejection fraction less than 50% |
| Left ventricular apical aneurysm |
| Extensive late gadolinium enhancement on cardiac magnetic resonance imaging |
| Nonsustained ventricular tachycardia on ambulatory monitoring |
Safety of Exercise
Moderate intensity exercise is safe and recommended in patients with stable HCM. Studies suggest a similar burden of ventricular arrhythmias in patients with HCM engaged in competitive sports as those who are not. Because the risk of sudden cardiac death is similar at rest and during competitive sports, vigorous exercise and sports may be reasonable with shared decision-making because no method of stratifying individual risks has been found. Placement of an implantable cardiac defibrillator to allow for athletic participation is not recommended.
Occupational Recommendations
Federal rules allow certification of truck drivers with HCM who do not have an implantable cardiac defibrillator or major risk factors of sudden cardiac death. Patients with asymptomatic HCM who are at low risk of sudden cardiac death and able to complete a treadmill stress test at 85% peak heart rate can be recommended as commercial airline pilots.
Pregnancy
Pregnancy is generally safe when HCM is stable. About one-fourth of patients experience cardiac symptoms, but sudden cardiac death during pregnancy and delivery is exceedingly rare. Most beta blockers other than atenolol are considered safe in pregnancy. Adverse outcomes occur in 3% to 4% of patients during delivery and are similar between vaginal and cesarean deliveries.
Editor's Note: Although the previous ACC/AHA guideline for hypertrophic cardiomyopathy was released 10 years ago, many of the new recommendations are unchanged. Atrioventricular blockade with beta blockers or nondihydropyridine calcium channel blockers remain the primary therapy for symptoms, and disopyramide and septal reduction remain secondary with slightly modified criteria. The most important change may be the new recommendations for moderate exercise and occupational certification for driving and flying. This guideline expands on the discussion of hypertrophic cardiomyopathy from the most recent article on cardiomyopathies (https://www.aafp.org/afp/2017/1115/p640.html).—Michael J. Arnold, MD, Contributing Editor
The views expressed are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, Uniformed Services University of the Health Sciences, Department of Defense, or the U.S. government.
Guideline source: American College of Cardiology / American Heart Association
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: Circulation. December 22, 2020;142(25):e558–e631
