Am Fam Physician. 2022;105(4):430-431
Author disclosure: No relevant financial relationships.
Clinical Question
Are selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) more effective for the treatment of vasomotor symptoms of menopause?
Evidence-Based Answer
Both SSRIs and SNRIs are effective at relieving vasomotor symptoms of menopause. (Strength of Recommendation [SOR]: A, systematic reviews.) No studies have directly compared the two classes of medication. SNRIs are associated with more adverse effects. Venlafaxine is preferred in women with breast cancer because SSRIs may interfere with tamoxifen metabolism. (SOR: C, expert opinion.)
Evidence Summary
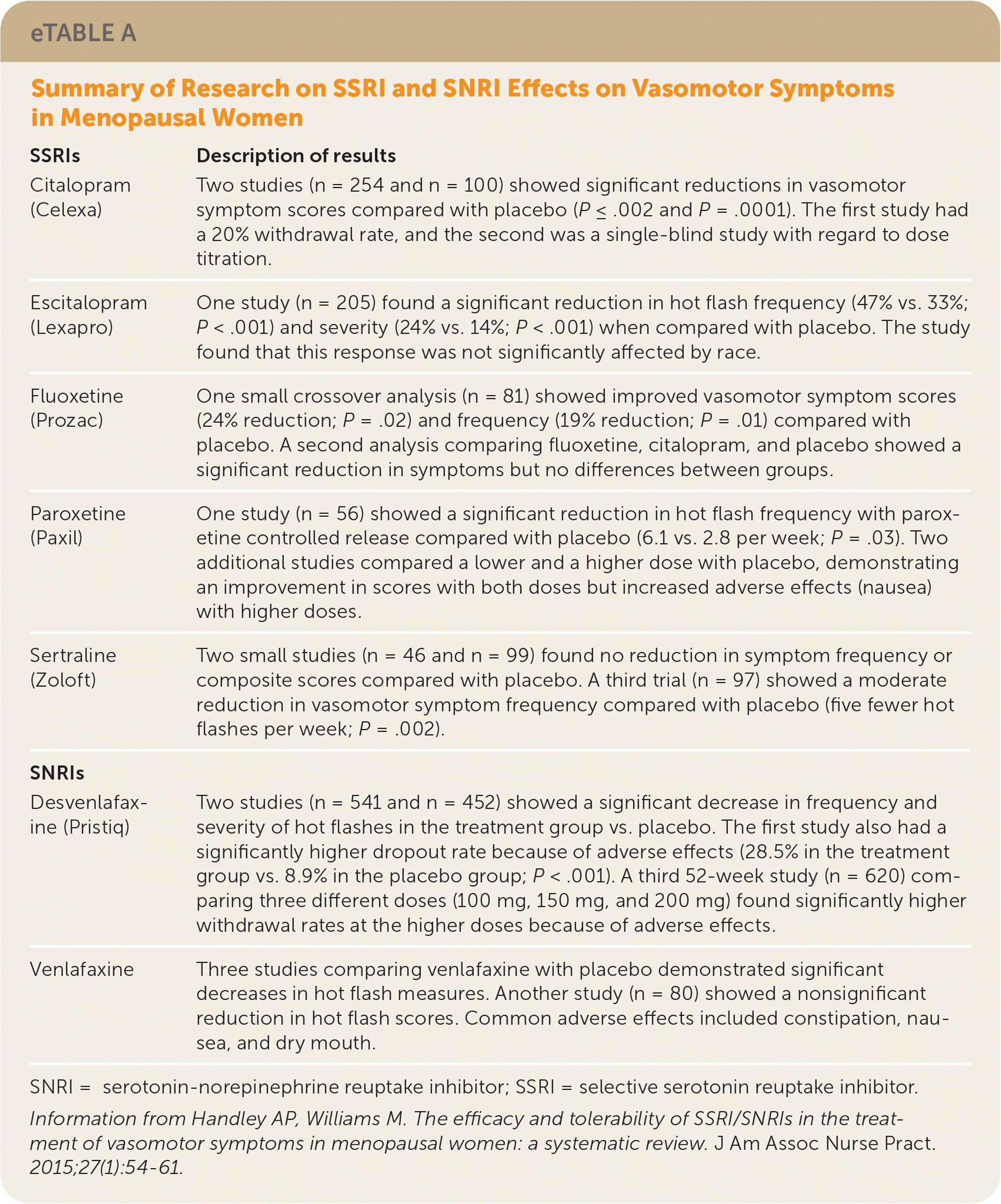
A 2015 systematic review of 18 randomized controlled trials (RCTs) involving women 27 to 78 years of age (N = 3,490) evaluated the effectiveness of SSRIs and SNRIs for the treatment of vasomotor symptoms in menopause.1 Study participants reported 0 to 50 hot flashes per week. Several studies included women with a history of cancer and stable selective estrogen receptor modulator use, but patients with active cancer were excluded. Patients using hormone therapy, antidepressants, and psychoactive drugs were also excluded. All studies evaluated vasomotor symptom frequency, measured as times per week, and average severity, rated on a scale of 0 to 4 (with 4 being the most severe). These measures were then multiplied to produce a composite score, with higher scores indicating more severe symptoms. Venlafaxine (37.5 mg) was identified as a first-line agent in the SNRI class. It showed the fastest onset of symptom relief (41% reduction alone by one week, 26% reduction vs. placebo; P < .001) but also more frequent adverse effects such as nausea, dry mouth, and constipation. The SSRI paroxetine (Paxil) showed the greatest overall reduction in hot flashes (40.6% at 10 mg and 51.7% at 20 mg; P = .0006 and P = .002, respectively) across both classes when compared with placebo. Additional SSRI and SNRI studies are described in eTable A. Limitations of this review included a primarily White population and multiple scales used to assess patient response.
| SSRIs | Description of results |
| Citalopram (Celexa) | Two studies (n = 254 and n = 100) showed significant reductions in vasomotor symptom scores compared with placebo (P ≤ .002 and P = .0001). The first study had a 20% withdrawal rate, and the second was a single-blind study with regard to dose titration. |
| Escitalopram (Lexapro) | One study (n = 205) found a significant reduction in hot flash frequency (47% vs. 33%; P < .001) and severity (24% vs. 14%; P < .001) when compared with placebo. The study found that this response was not significantly affected by race. |
| Fluoxetine (Prozac) | One small crossover analysis (n = 81) showed improved vasomotor symptom scores (24% reduction; P = .02) and frequency (19% reduction; P = .01) compared with placebo. A second analysis comparing fluoxetine, citalopram, and placebo showed a significant reduction in symptoms but no differences between groups. |
| Paroxetine (Paxil) | One study (n = 56) showed a significant reduction in hot flash frequency with paroxetine controlled release compared with placebo (6.1 vs. 2.8 per week; P = .03). Two additional studies compared a lower and a higher dose with placebo, demonstrating an improvement in scores with both doses but increased adverse effects (nausea) with higher doses. |
| Sertraline (Zoloft) | Two small studies (n = 46 and n = 99) found no reduction in symptom frequency or composite scores compared with placebo. A third trial (n = 97) showed a moderate reduction in vasomotor symptom frequency compared with placebo (five fewer hot flashes per week; P = .002). |
| SNRIs | |
| Desvenlafaxine (Pristiq) | Two studies (n = 541 and n = 452) showed a significant decrease in frequency and severity of hot flashes in the treatment group vs. placebo. The first study also had a significantly higher dropout rate because of adverse effects (28.5% in the treatment group vs. 8.9% in the placebo group; P < .001). A third 52-week study (n = 620) comparing three different doses (100 mg, 150 mg, and 200 mg) found significantly higher withdrawal rates at the higher doses because of adverse effects. |
| Venlafaxine | Three studies comparing venlafaxine with placebo demonstrated significant decreases in hot flash measures. Another study (n = 80) showed a nonsignificant reduction in hot flash scores. Common adverse effects included constipation, nausea, and dry mouth. |
A 2020 pooled analysis of four RCTs (N = 1,005) compared six interventions, including SSRIs and SNRIs, and their impact on vasomotor symptoms in peri- and postmenopausal women 40 to 62 years of age.2 The first trial (n = 205) was a two-arm, randomized, double-blind study comparing escitalopram (Lexapro; 10 or 20 mg) with placebo. Participants rated the frequency and impairment caused by vasomotor symptoms on a Likert scale, ranging from a score of 1 (no symptoms or impairment) to 8 (symptoms extremely bothersome). Women taking escitalopram reported a change of –0.4 points in vasomotor symptoms (95% CI, −0.9 to 0) at four weeks and −0.6 points (95% CI, −1.1 to −0.2) at eight weeks. A separate trial (n = 339) compared estradiol or venlafaxine extended-release (titrated from 37.5 mg to 75 mg over one week) with placebo. Estradiol showed the greatest reduction in vasomotor symptoms, followed by venlafaxine with a mean change of −0.3 points (95% CI, −0.7 to 0.2) at four weeks and −0.2 points (95% CI, −0.7 to 0.2) at eight weeks. SSRI and SNRI therapies were not compared with each other. Limitations included stricter inclusion criteria and lower baseline quality of life scores for the patients receiving SSRIs compared with those receiving SNRIs. It is also possible that higher baseline vasomotor symptom scores in the estradiol group resulted in a greater apparent change in self-reported symptoms.
A 2015 evidence-based position paper from the North American Menopause Society reviewed several different nonhormonal options for the treatment of perimenopausal vasomotor symptoms.3 The society concluded that both SSRIs and SNRIs provided significant relief of physiologic and surgical menopausal symptoms. It noted that paroxetine (10 to 25 mg per day), escitalopram (10 to 20 mg per day), and citalopram (Celexa; 10 to 20 mg per day) produced the largest reduction of symptoms among SSRIs, whereas the SNRIs that had significant effect were venlafaxine (37.5 to 150 mg per day) and desvenlafaxine (Pristiq; 100 to 150 mg per day). SSRIs were noted to have fewer initial adverse effects (specifically nausea and dizziness) but impaired tamoxifen metabolism. For this reason, venlafaxine was preferred in women with a history of breast cancer.