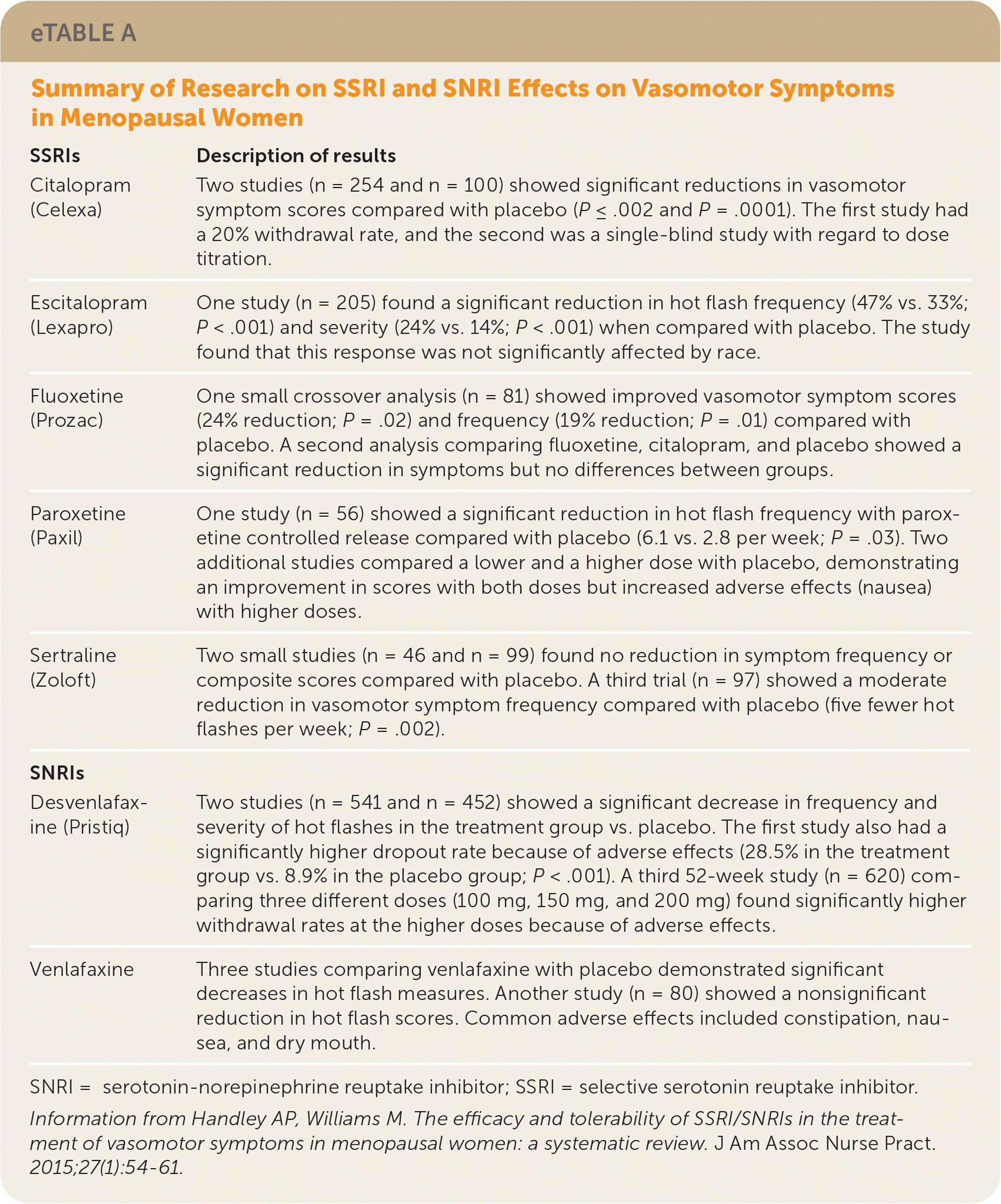
| SSRIs | Description of results |
| Citalopram (Celexa) | Two studies (n = 254 and n = 100) showed significant reductions in vasomotor symptom scores compared with placebo (P ≤ .002 and P = .0001). The first study had a 20% withdrawal rate, and the second was a single-blind study with regard to dose titration. |
| Escitalopram (Lexapro) | One study (n = 205) found a significant reduction in hot flash frequency (47% vs. 33%; P < .001) and severity (24% vs. 14%; P < .001) when compared with placebo. The study found that this response was not significantly affected by race. |
| Fluoxetine (Prozac) | One small crossover analysis (n = 81) showed improved vasomotor symptom scores (24% reduction; P = .02) and frequency (19% reduction; P = .01) compared with placebo. A second analysis comparing fluoxetine, citalopram, and placebo showed a significant reduction in symptoms but no differences between groups. |
| Paroxetine (Paxil) | One study (n = 56) showed a significant reduction in hot flash frequency with paroxetine controlled release compared with placebo (6.1 vs. 2.8 per week; P = .03). Two additional studies compared a lower and a higher dose with placebo, demonstrating an improvement in scores with both doses but increased adverse effects (nausea) with higher doses. |
| Sertraline (Zoloft) | Two small studies (n = 46 and n = 99) found no reduction in symptom frequency or composite scores compared with placebo. A third trial (n = 97) showed a moderate reduction in vasomotor symptom frequency compared with placebo (five fewer hot flashes per week; P = .002). |
| SNRIs | |
| Desvenlafaxine (Pristiq) | Two studies (n = 541 and n = 452) showed a significant decrease in frequency and severity of hot flashes in the treatment group vs. placebo. The first study also had a significantly higher dropout rate because of adverse effects (28.5% in the treatment group vs. 8.9% in the placebo group; P < .001). A third 52-week study (n = 620) comparing three different doses (100 mg, 150 mg, and 200 mg) found significantly higher withdrawal rates at the higher doses because of adverse effects. |
| Venlafaxine | Three studies comparing venlafaxine with placebo demonstrated significant decreases in hot flash measures. Another study (n = 80) showed a nonsignificant reduction in hot flash scores. Common adverse effects included constipation, nausea, and dry mouth. |