
Am Fam Physician. 2022;105(6):569-570
Published online April 19, 2022.
Author disclosure: No relevant financial relationships.
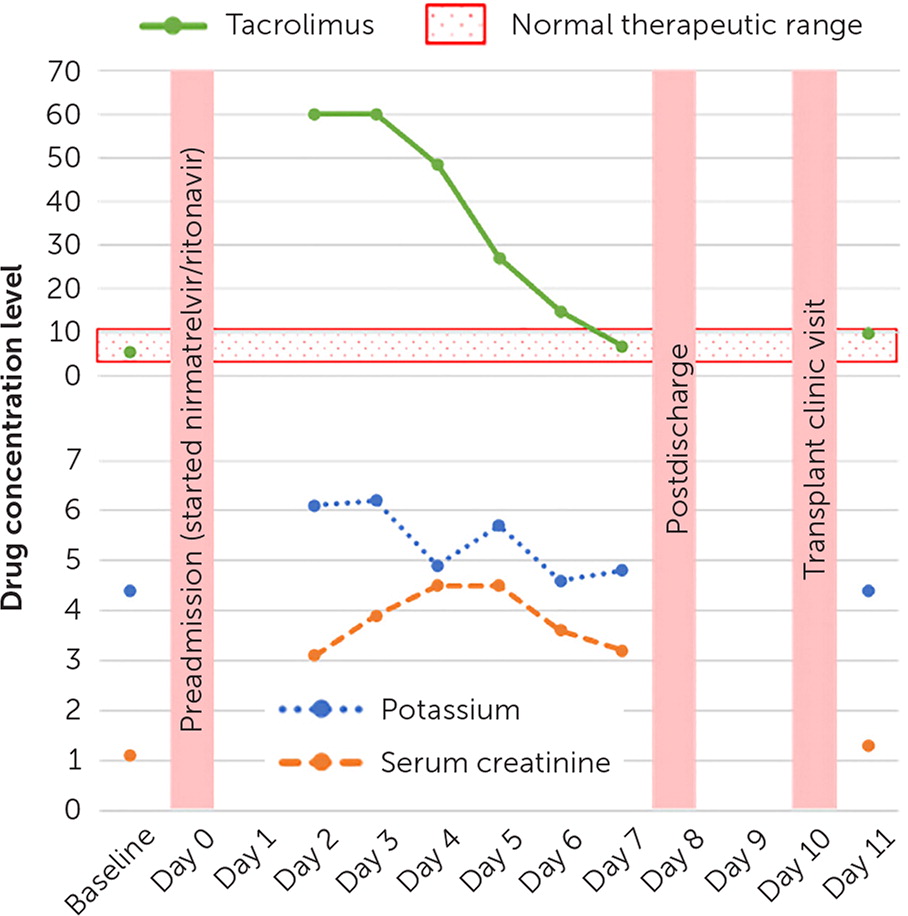
To the Editor: A 41-year-old female patient with a history of renal transplant presented to the emergency department with nausea, vomiting, and tremors. The patient had been prescribed nirmatrelvir/ritonavir (Paxlovid) for COVID-19 and had taken five of the 10 total doses. The patient was also taking 4 mg of extended-release tacrolimus (Envarsus) per day. Initial laboratory results showed an acute renal injury and hyperkalemia. The tacrolimus level was more than 60 ng per mL (therapeutic range is 4 to 10 ng per mL); therefore, tacrolimus and nirmatrelvir/ritonavir were held.
In December 2021, the U.S. Food and Drug Administration granted emergency use authorization for the first oral antiviral drug treatment for COVID-19 (Paxlovid).1 Nirmatrelvir/ritonavir is a combination of nirmatrelvir, a SARS-CoV-2 main protease inhibitor, and ritonavir, a potent cytochrome P-450 3A and P-glycoprotein inhibitor.2 The emergency use authorization is for patients who have mild to moderate COVID-19 who are at high risk of progression to severe COVID-19.2
Solid organ transplant recipients are an at-risk population because they are prescribed immunosuppressant medications from the calcineurin inhibitor class (e.g., tacrolimus, cyclosporine) or mammalian target of rapamycin inhibitor class (e.g., everolimus, sirolimus).3,4 All are substrates of cytochrome P-450 3A and P-glycoprotein. Concomitant administration of tacrolimus and ritonavir can slow the rate of tacrolimus metabolism, increasing its blood concentration to toxic levels.
