Am Fam Physician. 2022;106(3):online
Author disclosure: No relevant financial relationships.

Details for This Review
Study Population: Any patient receiving intravenous iron, oral iron, or placebo
Efficacy End Points: Dichotomous outcomes included treatment response (defined as an increase in hemoglobin of greater than 2 g per dL [20 g per L]), short-term mortality (30 days or less), long-term mortality (greater than 30 days), and requirement for a red blood cell transfusion; continuous outcomes included the mean difference in hemoglobin, number of units of red blood cells transfused, and length of hospital stay
Harm End Points: Proportion of patients who developed an infection
| Green | Benefits greater than harms |
| Yellow | Unclear benefits |
| Red | No benefits |
| Black | Harms greater than benefits |
Narrative: Iron deficiency anemia clinically affects 5 million people in the United States, with an additional 5 million who are deficient but asymptomatic.1 Patients considered at higher risk of developing iron deficiency anemia include infants and children younger than five years and those who are menstruating, are pregnant, or have chronic kidney disease or malabsorption syndromes.2
Intravenous iron infusions have emerged as an effective treatment for iron deficiency anemia. Antiquated formulations using high-molecular-weight iron dextran commonly caused hypersensitivity reactions; however, modern preparations such as iron sucrose, ferumoxytol (Feraheme), and sodium ferric gluconate (Ferrlecit) have shown fewer adverse effects.3,4 Evidence suggests that these modern formulations replete iron stores faster than oral therapy and can be transfused during one visit. Therefore, intravenous iron is now commonly prescribed for patients in high-risk groups, who cannot tolerate oral formulations, or who need expedited replenishment of iron (e.g., people in their third trimester of pregnancy, preoperative patients).2,5
The harms of intravenous iron infusions are not well documented. The risk of an anaphylactic reaction has decreased with modern formulations, but self-limited infusion reactions continue to occur.6 There is a risk of infection with iron supplementation, which may be secondary to iron's role as a pathogen growth and development factor. Studies using oral iron have shown an increased risk of malaria and diarrheal diseases in endemic areas.7 Intravenous iron is postulated to have similar risks, but trials have shown varied effects. Most studies do not report infections as outcomes; others are underpowered to detect harm differences.
Specialty guidelines address infections only as potential contraindications for intravenous iron infusions. The International Society of Nephrology recommends against administering intravenous iron in patients with active infections (no evidence grade), and the British Society of Gastroenterology recommends caution in using parenteral iron during acute and chronic infections (no evidence grade).8,9
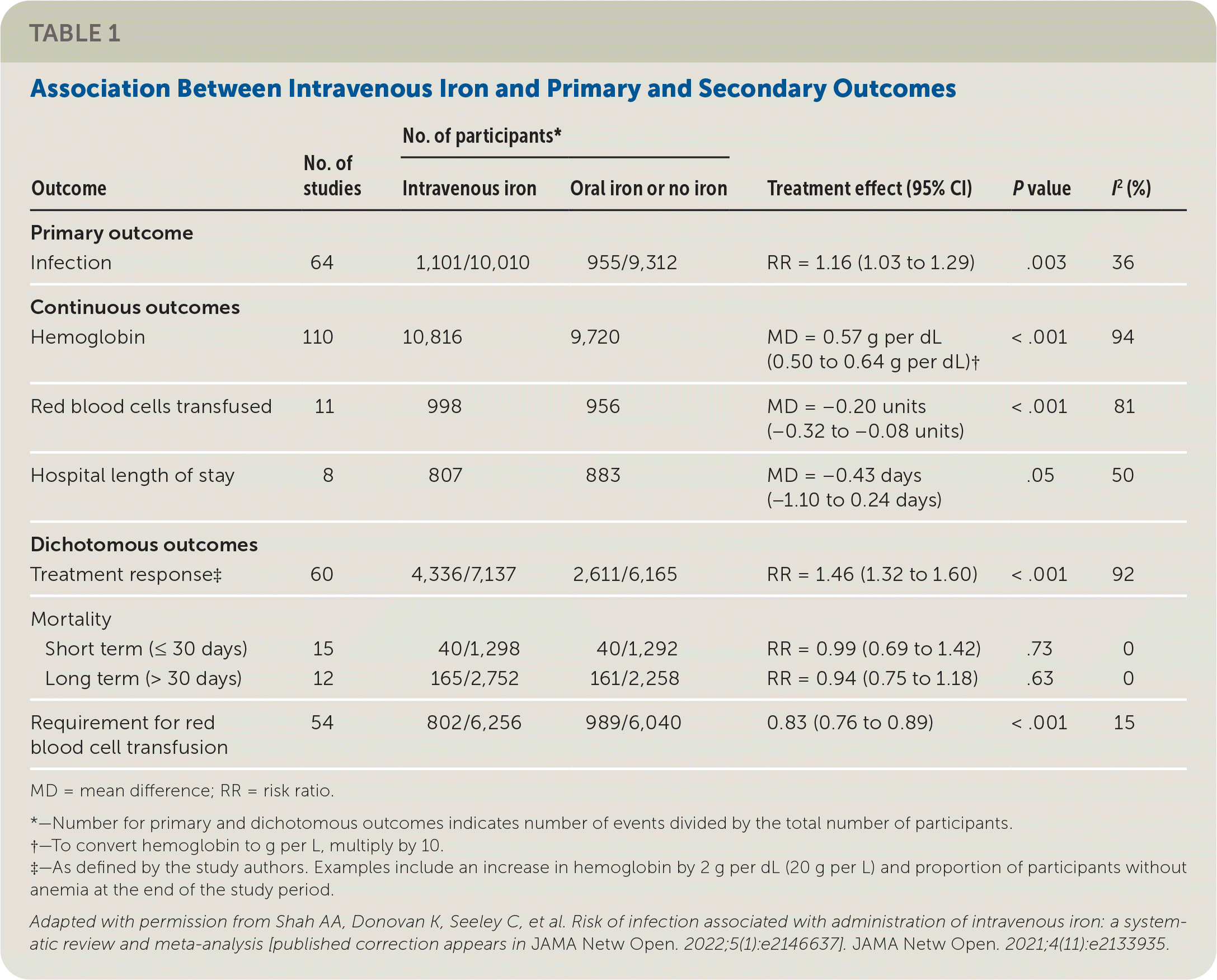
This systematic review and meta-analysis assessed the risk of infection from intravenous iron and the intended beneficial effects on hemoglobin levels, red blood cell transfusion requirements, and mortality (Table 1).10 The authors identified 154 randomized controlled trials, with approximately 32,762 patients, that compared intravenous iron with oral iron or placebo. The review was not intended to assess the effectiveness of intravenous iron for the treatment of iron deficiency anemia because inclusion criteria specified the use of parenteral or oral iron but not a diagnosis of anemia.
| Outcome | No. of studies | No. of participants* | Treatment effect (95% CI) | P value | I2 (%) | |
|---|---|---|---|---|---|---|
| Intravenous iron | Oral iron or no iron | |||||
| Primary outcome | ||||||
| Infection | 64 | 1,101/10,010 | 955/9,312 | RR = 1.16 (1.03 to 1.29) | .003 | 36 |
| Continuous outcomes | ||||||
| Hemoglobin | 110 | 10,816 | 9,720 | MD = 0.57 g per dL (0.50 to 0.64 g per dL)† | < .001 | 94 |
| Red blood cells transfused | 11 | 998 | 956 | MD = −0.20 units (−0.32 to −0.08 units) | < .001 | 81 |
| Hospital length of stay | 8 | 807 | 883 | MD = −0.43 days (−1.10 to 0.24 days) | .05 | 50 |
| Dichotomous outcomes | ||||||
| Treatment response‡ | 60 | 4,336/7,137 | 2,611/6,165 | RR = 1.46 (1.32 to 1.60) | < .001 | 92 |
| Mortality | ||||||
| Short term (≤ 30 days) | 15 | 40/1,298 | 40/1,292 | RR = 0.99 (0.69 to 1.42) | .73 | 0 |
| Long term (> 30 days) | 12 | 165/2,752 | 161/2,258 | RR = 0.94 (0.75 to 1.18) | .63 | 0 |
| Requirement for red blood cell transfusion | 54 | 802/6,256 | 989/6,040 | 0.83 (0.76 to 0.89) | < .001 | 15 |
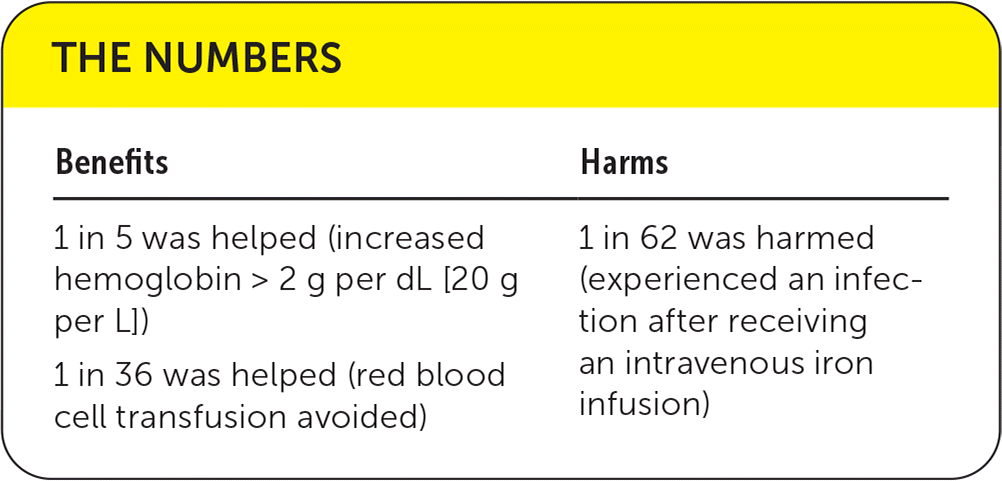
Intravenous iron was associated with a significantly increased risk of infection compared with oral iron or no iron (relative risk [RR] = 1.16; 95% CI, 1.03 to 1.29; absolute risk difference [ARD] = 1.64; number needed to harm = 62). Genitourinary and lung infections were most common, but reporting and definitions of infections varied across studies. A subgroup analysis identified a higher risk of infection in patients with inflammatory bowel disease (RR = 1.73; 95% CI, 1.11 to 2.71). The type of iron formulation and the patient's baseline ferritin or hemoglobin level did not affect infection risks.
Patients receiving intravenous iron had a mean improvement in hemoglobin of 0.57 g per dL; 95% CI, 0.50 to 0.64 g per dL (5.7 g per L; 95% CI, 5.0 to 6.4 g per L) in addition to a dichotomous treatment response of a greater than 2 g per dL change in hemoglobin (RR = 1.46; 95% CI, 1.32 to 1.60; ARD = 19.48; 95% CI, 13.6 to 25.4) compared with oral or no iron. There was a smaller change in red blood cell units transfused, with a mean difference of −0.20 units (95% CI, −0.32 to −0.08 units) and a lower risk of any transfusion (RR = 0.83; 95% CI, 0.76 to 0.89; ARD = −2.8; 95% CI, −1.8 to −3.9) after intravenous iron infusion. Intravenous iron had no effect on short- or long-term mortality or length of hospital stay.
Caveats: The systematic review did not include other adverse effects from intravenous or oral iron, such as hypersensitivity reactions or gastrointestinal adverse effects. When studies at high risk of bias are removed, the higher RR of infection becomes nonsignificant. The authors report that this is likely due to varied reporting of infections across studies because only 13 of the 34 randomized controlled trials reviewed had prespecified infection-related outcomes.8 The systematic review also does not specify worsening of active infections with intravenous iron and reports only the incidence of new infections.
Only disease-specific outcomes were significant (hemoglobin level, red blood cell units transfused) when considering the benefits of intravenous iron. Patient-oriented outcomes, such as length of hospital stay and mortality, did not reach statistical significance but may have been underpowered. It is also possible that these outcomes were confounded by red blood cell transfusions administered at a certain threshold to prevent poor outcomes.
A yellow recommendation (unclear benefits) was selected because of the uncertain evidence of harm with limited evidence of improvement in patient-oriented outcomes (e.g., length of hospital stay, mortality) from intravenous iron compared with oral or no iron. The infection risk of intravenous iron is inconclusive at best. Per these data, approximately one patient will acquire an infection for every two red blood cell transfusions prevented; therefore, physicians must weigh the risk of infection against the benefit of avoiding a transfusion.