
Am Fam Physician. 2022;106(5):575-577
Author disclosure: No relevant financial relationships.
Binx IO is a cartridge-based test with a Clinical Laboratory Improvement Amendment (CLIA) waiver that detects Chlamydia trachomatis and Neisseria gonorrhoeae infections in patients with or without symptoms.1 The molecular assay is performed by clinicians using vaginal swabs or male urine specimens. Results are available in 30 minutes, allowing for testing and treatment in a single visit.1
Accuracy
A 2020 industry-funded, cross-sectional study of 1,523 women and 922 men presenting to sexually transmitted infection, HIV, family planning, or obstetrics and gynecology clinics compared the performance of Binx IO with three commercially available nucleic acid amplification tests (NAATs).2 Patients who took antibiotics effective against chlamydia or gonorrhea within the previous 28 days were excluded. Symptoms were reported in 54% of women and 33% of men, including abnormal discharge, dysuria, genital itching, pelvic pain, and discomfort with intercourse. Four vaginal swab samples were collected from women. Three of the samples were collected by a clinician and tested with NAATs. The fourth vaginal sample was tested with Binx IO, with one-half of these vaginal swabs collected by the patient and one-half by the clinician. A first-catch urine sample was collected from men, then divided into four transport devices (three tested with NAATs and one with Binx IO).
Patients were classified as infected if at least two of the three NAAT results were positive. The overall prevalence was 8.5% in women and 13% in men for chlamydia, and 3% in women and 8% in men for gonorrhea. Sensitivity and specificity of Binx IO were greater than 90% for vaginal samples and male urine samples. Test performance is summarized in Table 1 and Table 2.2
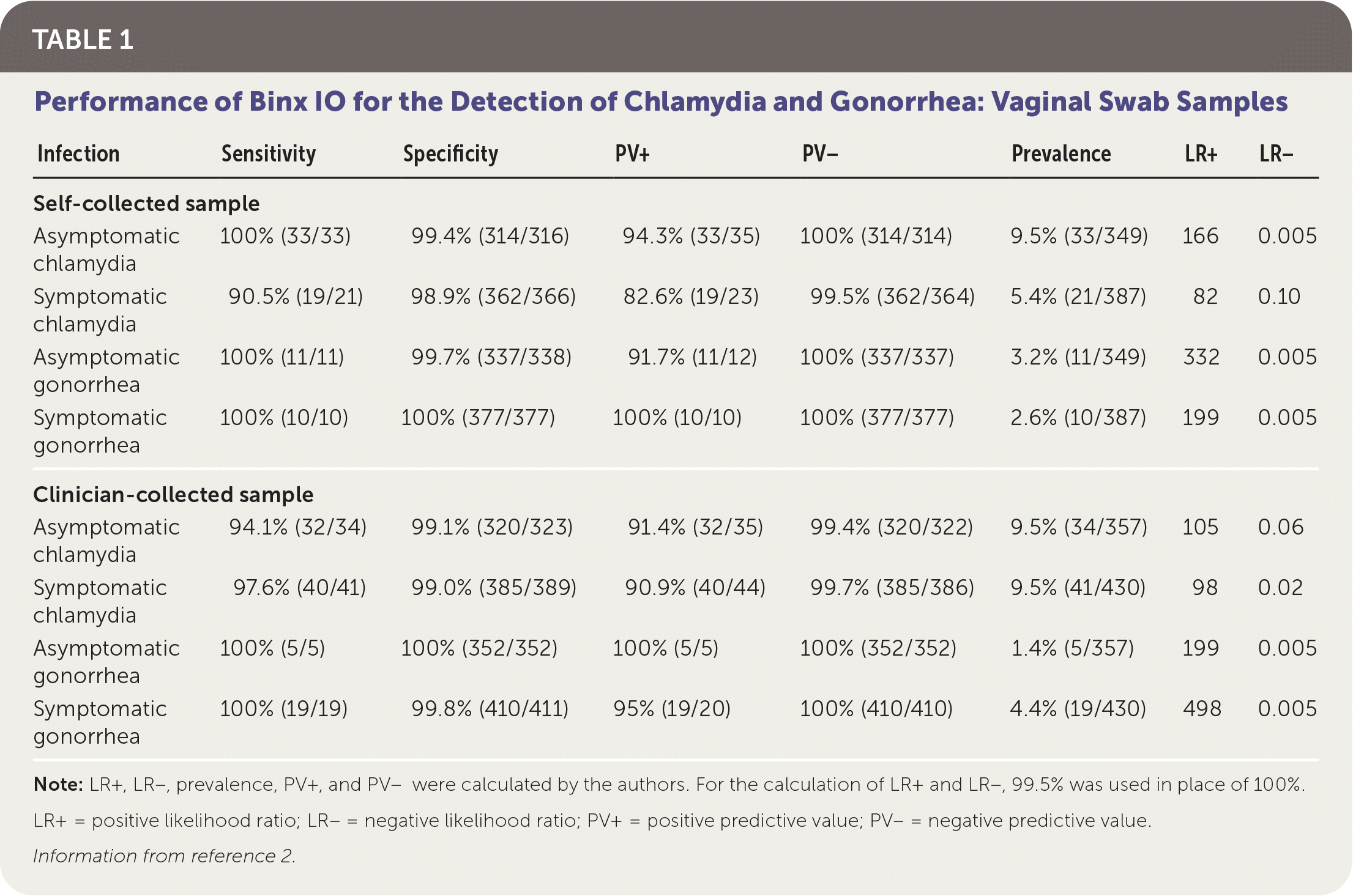
| Infection | Sensitivity | Specificity | PV+ | PV− | Prevalence | LR+ | LR− |
|---|---|---|---|---|---|---|---|
| Self-collected sample | |||||||
| Asymptomatic chlamydia | 100% (33/33) | 99.4% (314/316) | 94.3% (33/35) | 100% (314/314) | 9.5% (33/349) | 166 | 0.005 |
| Symptomatic chlamydia | 90.5% (19/21) | 98.9% (362/366) | 82.6% (19/23) | 99.5% (362/364) | 5.4% (21/387) | 82 | 0.10 |
| Asymptomatic gonorrhea | 100% (11/11) | 99.7% (337/338) | 91.7% (11/12) | 100% (337/337) | 3.2% (11/349) | 332 | 0.005 |
| Symptomatic gonorrhea | 100% (10/10) | 100% (377/377) | 100% (10/10) | 100% (377/377) | 2.6% (10/387) | 199 | 0.005 |
| Clinician-collected sample | |||||||
| Asymptomatic chlamydia | 94.1% (32/34) | 99.1% (320/323) | 91.4% (32/35) | 99.4% (320/322) | 9.5% (34/357) | 105 | 0.06 |
| Symptomatic chlamydia | 97.6% (40/41) | 99.0% (385/389) | 90.9% (40/44) | 99.7% (385/386) | 9.5% (41/430) | 98 | 0.02 |
| Asymptomatic gonorrhea | 100% (5/5) | 100% (352/352) | 100% (5/5) | 100% (352/352) | 1.4% (5/357) | 199 | 0.005 |
| Symptomatic gonorrhea | 100% (19/19) | 99.8% (410/411) | 95% (19/20) | 100% (410/410) | 4.4% (19/430) | 498 | 0.005 |
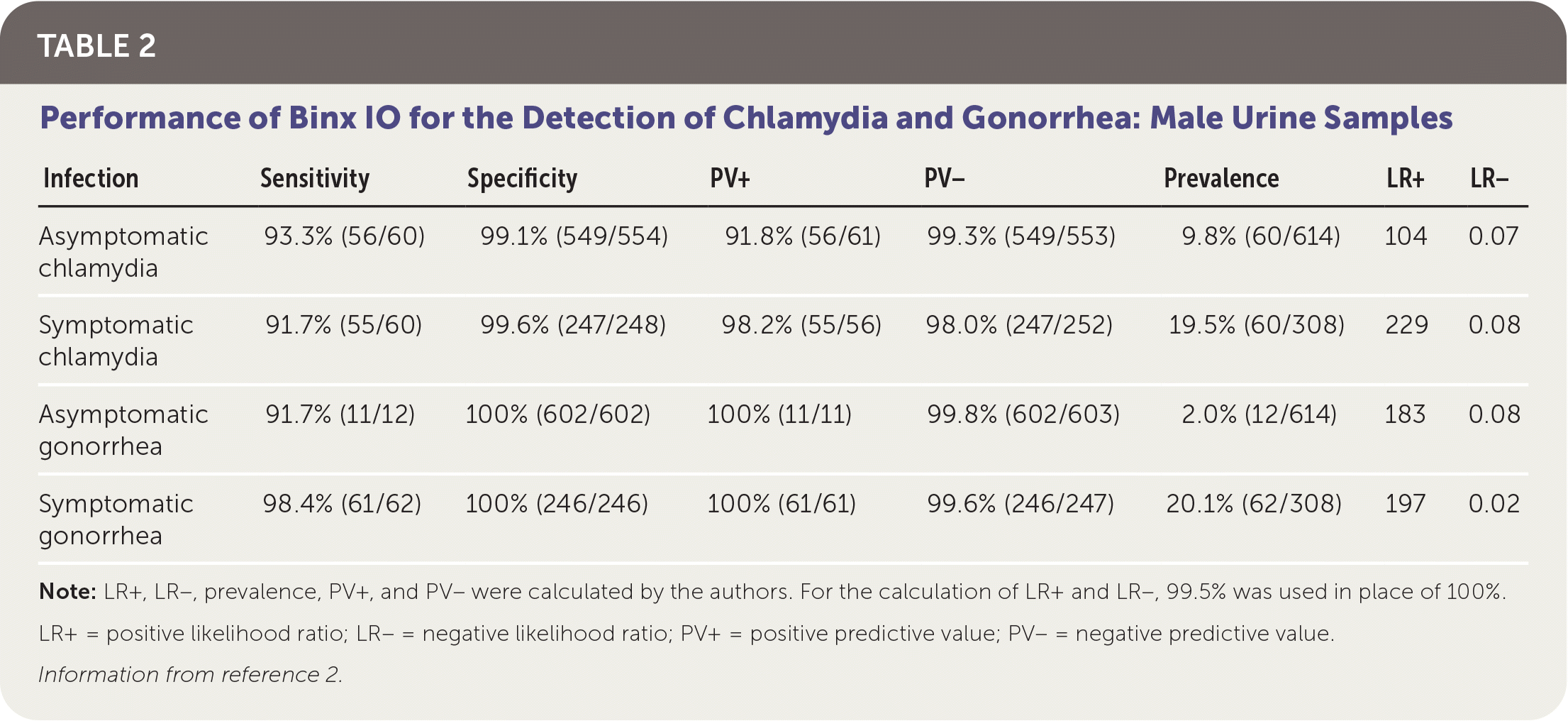
| Infection | Sensitivity | Specificity | PV+ | PV− | Prevalence | LR+ | LR− |
|---|---|---|---|---|---|---|---|
| Asymptomatic chlamydia | 93.3% (56/60) | 99.1% (549/554) | 91.8% (56/61) | 99.3% (549/553) | 9.8% (60/614) | 104 | 0.07 |
| Symptomatic chlamydia | 91.7% (55/60) | 99.6% (247/248) | 98.2% (55/56) | 98.0% (247/252) | 19.5% (60/308) | 229 | 0.08 |
| Asymptomatic gonorrhea | 91.7% (11/12) | 100% (602/602) | 100% (11/11) | 99.8% (602/603) | 2.0% (12/614) | 183 | 0.08 |
| Symptomatic gonorrhea | 98.4% (61/62) | 100% (246/246) | 100% (61/61) | 99.6% (246/247) | 20.1% (62/308) | 197 | 0.02 |
Benefits
Binx IO is a CLIA-waived test requiring only five minutes of training and less than one minute of total hands-on time to run the test, with results available in 30 minutes.1,2 The ability to self-collect vaginal swabs may increase acceptability for patients who prefer this method and do not otherwise need a pelvic examination.
Increasing on-site testing allows for rapid treatment and potentially prevents transmission to partners. With current NAATs, which provide results in one to two days, patients who are not treated empirically during initial presentation may not return for treatment once results become available. Binx IO also has the potential to improve antibiotic stewardship by reducing unnecessary antibiotic use in patients who receive empiric therapy before NAAT test results become available.
Harms
Further evaluation of Binx IO is needed to validate testing of rectal and oropharyngeal samples and female urine samples. In men who have sex with men, approximately 75% of extragenital N. gonorrhoeae infections and 89% of extragenital C. trachomatis infections occur with negative urogenital NAAT results; therefore, validating these additional testing sites for Binx IO is important.3 The sensitivity of Binx IO for detecting chlamydia using male urine samples was 92.5%, with a specificity of 99%. The slightly lower sensitivity of testing using urine specimens may potentially lead to false-negative results and delayed treatment.2
Cost
Binx IO is reimbursed at a rate of $70.20 under Current Procedural Terminology (CPT) code 87801 QW.4 This is similar to NAATs without the CLIA waiver. If testing for gonorrhea or chlamydia alone, costs for other amplification probe tests are reimbursed at $35.09.4 The full cost associated with the Binx IO is unclear because pricing for the testing instrument and cartridges is not publicly available.1
Bottom Line
Binx IO is a rapid, point-of-care option for the detection of C. trachomatis and N. gonorrhoeae infections in adults and can be used for screening and diagnosis. The test can be completed in approximately 30 minutes, allowing for testing and treatment in the same visit. Limitations include slightly lower sensitivity with male urine samples for the detection of chlamydia and unknown performance with anal, oropharyngeal, or female urine samples.
