Am Fam Physician. 2022;106(6):628-636
Patient information: See related handout on when antibiotics can help with upper respiratory infections.
Published online November 7, 2022.
Author disclosure: No relevant financial affiliations.
Upper respiratory tract infections are responsible for millions of physician visits in the United States annually. Although viruses cause most acute upper respiratory tract infections, studies show that many infections are unnecessarily treated with antibiotics. Because inappropriate antibiotic use results in adverse events, contributes to antibiotic resistance, and adds unnecessary costs, family physicians must take an evidence-based, judicious approach to the use of antibiotics in patients with upper respiratory tract infections. Antibiotics should not be used for the common cold, influenza, COVID-19, or laryngitis. Evidence supports antibiotic use in most cases of acute otitis media, group A beta-hemolytic streptococcal pharyngitis, and epiglottitis and in a limited percentage of acute rhinosinusitis cases. Several evidence-based strategies have been identified to improve the appropriateness of antibiotic prescribing for acute upper respiratory tract infections.
Upper respiratory tract infections (URIs) account for millions of physician visits in the United States annually.1 Most URIs are caused by viruses and do not require antibiotics; however, studies show that up to 10 million antibiotic prescriptions per year are inappropriately directed toward respiratory tract infections.2 One cohort study of approximately 15,000 outpatients with acute URIs found that 41% of patients prescribed antibiotics did not have an indication for them.3 Unnecessary antibiotic use results in adverse events, contributes to antibiotic resistance, and adds unnecessary costs. Adverse events are usually mild (e.g., diarrhea, rash) but can be more severe (e.g., Stevens-Johnson syndrome, Clostridioides difficile colitis) or even life-threatening (e.g., anaphylaxis, sudden cardiac death).4 Although it is important to recognize conditions that should not be treated with antibiotics, it is also important to recognize URIs that almost always require antibiotics (Table 14–19). Family physicians should familiarize themselves with an evidence-based approach to antibiotic use for URIs to achieve the three goals of antibiotic stewardship: improve patient outcomes, minimize unintended consequences, and prevent unnecessary health care costs.
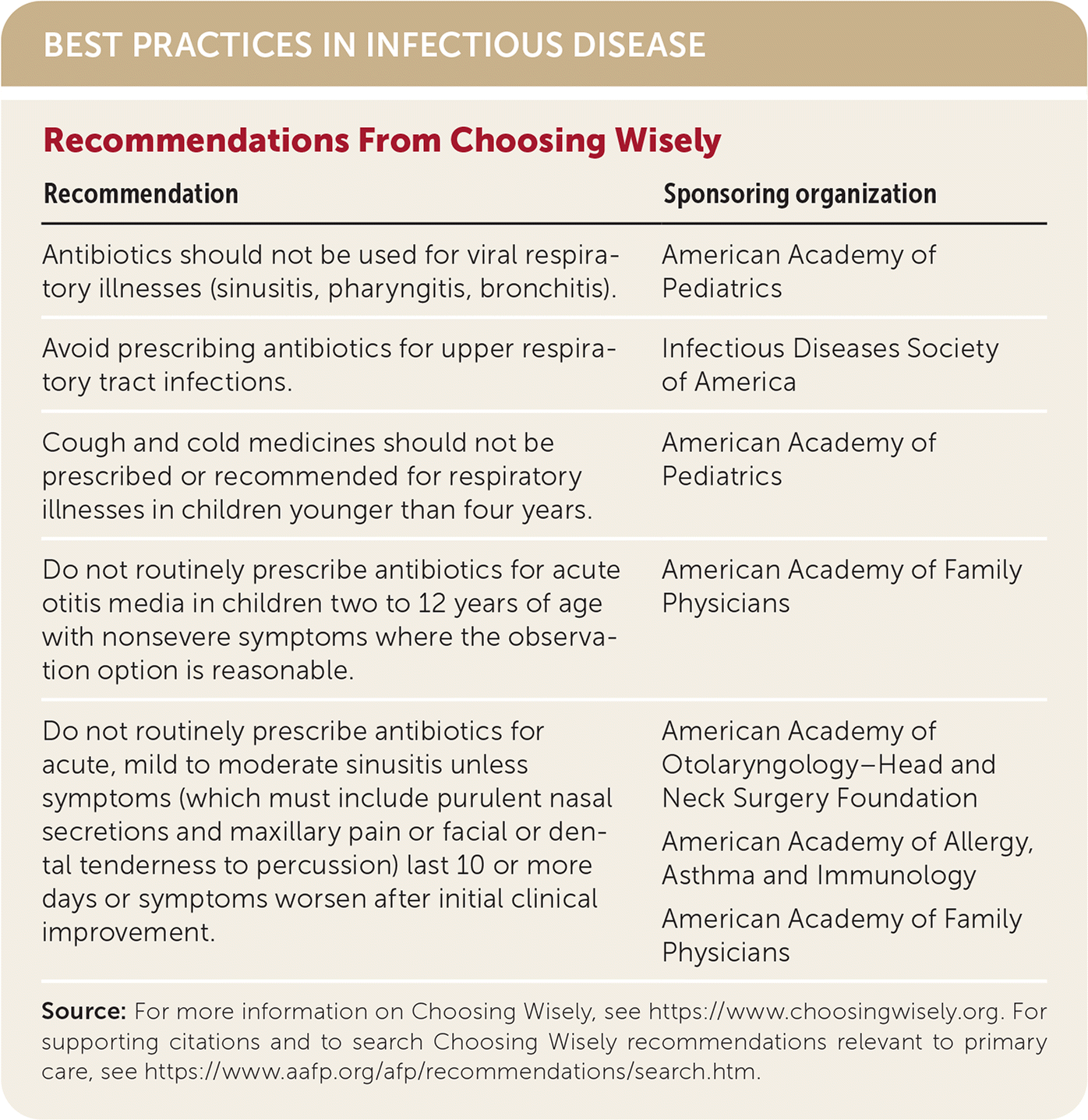
| Recommendation | Sponsoring organization |
|---|---|
| Antibiotics should not be used for viral respiratory illnesses (sinusitis, pharyngitis, bronchitis). | American Academy of Pediatrics |
| Avoid prescribing antibiotics for upper respiratory tract infections. | Infectious Diseases Society of America |
| Cough and cold medicines should not be prescribed or recommended for respiratory illnesses in children younger than four years. | American Academy of Pediatrics |
| Do not routinely prescribe antibiotics for acute otitis media in children two to 12 years of age with nonsevere symptoms where the observation option is reasonable. | American Academy of Family Physicians |
| Do not routinely prescribe antibiotics for acute, mild to moderate sinusitis unless symptoms (which must include purulent nasal secretions and maxillary pain or facial or dental tenderness to percussion) last 10 or more days or symptoms worsen after initial clinical improvement. | American Academy of Otolaryngology–Head and Neck Surgery Foundation American Academy of Allergy, Asthma and Immunology American Academy of Family Physicians |
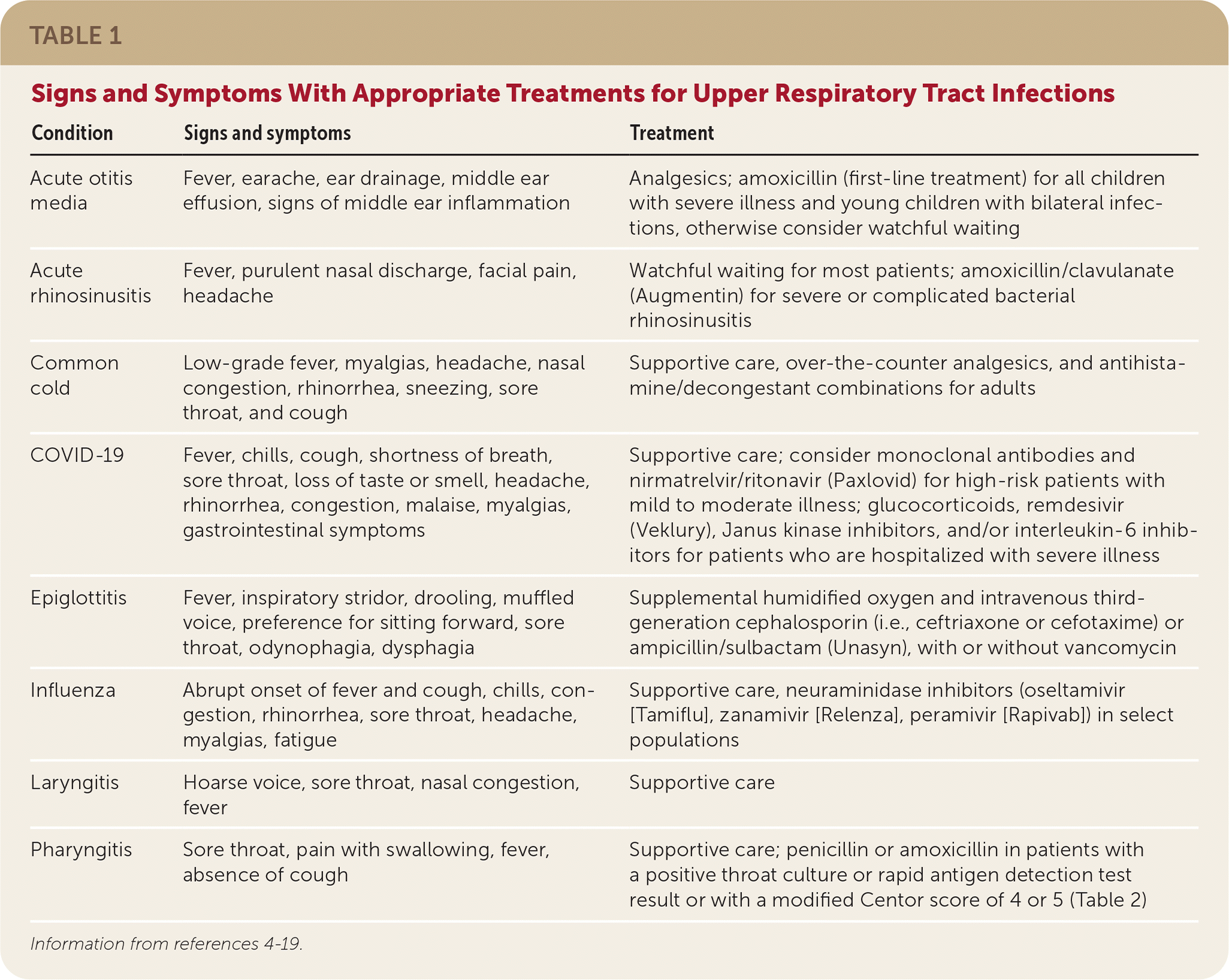
| Condition | Signs and symptoms | Treatment |
|---|---|---|
| Acute otitis media | Fever, earache, ear drainage, middle ear effusion, signs of middle ear inflammation | Analgesics; amoxicillin (first-line treatment) for all children with severe illness and young children with bilateral infections, otherwise consider watchful waiting |
| Acute rhinosinusitis | Fever, purulent nasal discharge, facial pain, headache | Watchful waiting for most patients; amoxicillin/clavulanate (Augmentin) for severe or complicated bacterial rhinosinusitis |
| Common cold | Low-grade fever, myalgias, headache, nasal congestion, rhinorrhea, sneezing, sore throat, and cough | Supportive care, over-the-counter analgesics, and antihistamine/decongestant combinations for adults |
| COVID-19 | Fever, chills, cough, shortness of breath, sore throat, loss of taste or smell, headache, rhinorrhea, congestion, malaise, myalgias, gastrointestinal symptoms | Supportive care; consider monoclonal antibodies and nirmatrelvir/ritonavir (Paxlovid) for high-risk patients with mild to moderate illness; glucocorticoids, remdesivir (Veklury), Janus kinase inhibitors, and/or interleukin-6 inhibitors for patients who are hospitalized with severe illness |
| Epiglottitis | Fever, inspiratory stridor, drooling, muffled voice, preference for sitting forward, sore throat, odynophagia, dysphagia | Supplemental humidified oxygen and intravenous third-generation cephalosporin (i.e., ceftriaxone or cefotaxime) or ampicillin/sulbactam (Unasyn), with or without vancomycin |
| Influenza | Abrupt onset of fever and cough, chills, congestion, rhinorrhea, sore throat, headache, myalgias, fatigue | Supportive care, neuraminidase inhibitors (oseltamivir [Tamiflu], zanamivir [Relenza], peramivir [Rapivab]) in select populations |
| Laryngitis | Hoarse voice, sore throat, nasal congestion, fever | Supportive care |
| Pharyngitis | Sore throat, pain with swallowing, fever, absence of cough | Supportive care; penicillin or amoxicillin in patients with a positive throat culture or rapid antigen detection test result or with a modified Centor score of 4 or 5 (Table 2) |
Common Cold
Although the common cold is typically a mild, self-limited upper respiratory viral illness, it significantly affects work and school attendance and productivity, and is commonly treated with antibiotics. Symptoms such as a low-grade fever, myalgias, headache, nasal congestion, rhinorrhea, sneezing, sore throat, and cough often last up to 10 days. Treatments with established effectiveness for those symptoms in adults are limited to over-the-counter analgesics and antihistamine/decongestant combinations.5 The American Academy of Pediatrics Choosing Wisely recommendation states that cough and cold medicines should be avoided for respiratory illness in children younger than four years.20 Antibiotics are ineffective for the treatment of the common cold in adults and children and should not be prescribed based on consistent findings of no benefit and increased adverse effects.5,21,22
Influenza
Symptoms of influenza include fever, chills, cough, congestion, runny nose, sore throat, headaches, myalgias, and fatigue. Influenza caused an estimated 9.3 million to 41 million illnesses, 4.3 million to 21 million medical visits, 140,000 to 710,000 hospitalizations, and 12,000 to 52,000 deaths annually between 2010 and 2020 in the United States.23
Annual vaccines can help prevent serious illness from influenza in patients six months and older with no contraindications. The influenza vaccine helps prevent antibiotic resistance by reducing the number of acute febrile illnesses caused by influenza that may be treated inappropriately with antibiotics and by decreasing the number of secondary bacterial infections that may require antibiotic therapy.24,25 Multiple studies have found a negative association between influenza vaccine coverage and antibiotic prescribing. A retrospective study on influenza vaccine coverage and antibiotic prescription rates between 2010 and 2017 in the United States concluded that a 10-point increase in influenza vaccination rates was associated with a 6.5% reduction in antibiotic prescription rates.24 Although antibiotics have no role in influenza treatment unless they are used to treat a secondary bacterial infection, appropriate antiviral use as soon as possible once the diagnosis is suspected or has been confirmed can decrease the duration of symptoms and the risk of severe complications.6,26
COVID-19
In 2021, COVID-19 was associated with approximately 460,000 deaths between January and December, which equated to 60,000 more deaths in 2021 compared with 2020.27 There have been more than 5 million total hospital admissions across all ages since 2020.28 COVID-19 typically causes mild to severe symptoms, including fever, chills, cough, shortness of breath, loss of taste or smell, headache, malaise, myalgias, sore throat, congestion, rhinorrhea, and gastrointestinal symptoms. Serious complications can result from COVID-19, such as pneumonia, acute respiratory distress syndrome, arrhythmias, multiorgan failure, septic shock, and death. Adults 65 years and older, patients with underlying medical conditions, patients who are pregnant or were recently pregnant, and people of racial and ethnic minorities are at the highest risk of developing these serious complications.29
Vaccines for SARS-CoV-2 have decreased the incidence of COVID-19 and prevented hospitalization and death in patients who develop symptomatic infections. Treatment is supportive; however, several therapies are available for patients who are at high risk or severely ill. Antibiotics are not recommended to prevent or treat COVID-19 unless a superimposed bacterial infection is suspected. The use of azithromycin (Zithromax) with hydroxychloroquine (Plaquenil) in patients with COVID-19 has been associated with an increased risk of QT prolongation without a clear mortality benefit.7
Laryngitis
Acute laryngitis is an inflammation of the larynx and vocal cords that clinically presents as a hoarse voice typically associated with other symptoms of URI. The treatment of acute laryngitis with antibiotics is usually unnecessary because the infectious source is viral, and the course is self-limited. A Cochrane review of laryngitis concluded that antibiotics are not typically effective for treatment, and any benefits, including a slight improvement in voice, do not outweigh the costs.8 Antibiotics in the treatment of laryngitis should be avoided.
Acute Otitis Media
Acute otitis media (AOM) is defined as the rapid onset of signs and symptoms of inflammation in the middle ear. AOM is the most common diagnosis leading to antibiotic prescriptions for children in the United States.11 Antibiotic treatment of AOM leads to modest improvement in symptoms compared with placebo or delayed antibiotics; however, between 4% and 10% of children treated with antibiotics for AOM experience adverse effects, primarily diarrhea and rash.12,30,31 Despite clear guidelines on the diagnosis and treatment of AOM, a correct diagnosis in infants and young children is difficult because symptoms can be mild, and proper visualization of the patient’s tympanic membranes can be limited by patient cooperation, cerumen in the ear canal, and the self-limiting nature of the disease. AOM is uncommon in adults, and guidelines on diagnosis and treatment in this population are lacking.
The diagnosis of AOM requires the presence of moderate to severe bulging of the tympanic membrane, new onset of otorrhea not due to acute otitis externa, or mild bulging of the tympanic membrane and recent (less than 48 hours) onset of ear pain or intense erythema of the tympanic membrane.11 Ear pain may manifest as tugging, rubbing, or holding the ear; irritability; or excessive crying.4,11 Middle ear effusion in the absence of clinical symptoms suggestive of AOM is defined as otitis media with effusion and does not require antibiotic therapy.11,32
Analgesics should be recommended in the management of all patients with AOM.11 Watchful waiting with a plan for rescue antibiotics should be considered in children two years and older with mild signs and symptoms and in children six to 23 months with mild, unilateral AOM.4,11 Through joint decision-making, parents can be provided a prescription during the initial visit to be filled if the child does not improve or worsens within two to three days. Alternatively, patients can be scheduled for a follow-up visit in two to three days with instructions to return earlier if symptoms worsen.4,11,12 Although watchful waiting in children with AOM may be associated with higher costs to patients and the health care system due to the additional physician visits, it has decreased the incidence of antibiotics initiation.33 Amoxicillin is the first-line treatment in children with AOM when the decision is made to treat with antibiotics and the child has not received amoxicillin in the past 30 days, does not have concurrent purulent conjunctivitis, and is not allergic to penicillin.4,11,12 Children with more severe disease (i.e., severe otalgia, otalgia lasting more than 48 hours, or a temperature of 102.2°F [39°C] or greater) and children 23 months or younger with bilateral infection are more likely to benefit from antibiotic treatment with high-dose amoxicillin.11 Children younger than two years and those with severe symptoms should receive a 10-day course of antibiotics, whereas a five- to seven-day course is sufficient in older children with mild to moderate symptoms.11
Group A Beta-Hemolytic Streptococcal Pharyngitis
Pharyngitis symptoms include sore throat, fever, and painful swallowing. Children may also have headaches, nausea, or abdominal pain. Pharyngitis is a self-limited infection that may accompany other symptoms of URIs. Although group A beta-hemolytic streptococcal pharyngitis is the most common bacterial cause of acute pharyngitis, accounting for 5% to 15% of sore throat visits in adults and 20% to 30% in children, most cases are viral.13 Distinguishing group A beta-hemolytic streptococcal pharyngitis from viral pharyngitis can be difficult based on clinical features alone. Group A beta-hemolytic streptococcal pharyngitis is more common in children five to 15 years of age than in adults. Treatment of group A beta-hemolytic streptococcal pharyngitis with appropriate antibiotics helps prevent the rare complications of acute rheumatic fever and peritonsillar abscess, hastens clinical resolution, and decreases contagion.4,13,14,34
The American Academy of Family Physicians, the American College of Physicians, and the Centers for Disease Control and Prevention (CDC) recommend using the modified Centor criteria when evaluating patients with pharyngitis to determine the likelihood of group A beta-hemolytic streptococcal infection before prescribing antibiotics (Table 235). The findings in the Centor criteria that are suggestive of group A beta-hemolytic streptococcal pharyngitis include tonsillar exudates, tender anterior cervical lymphadenopathy or lymphadenitis, absence of cough, and a history of fever. A score of 3 or 4 has a positive predictive value of 40% to 60% and a negative predictive value of approximately 80%.14 Because the Centor score is only applicable to adults, the modified Centor criteria (McIsaac score) was developed for use in children and includes age as a criterion.36
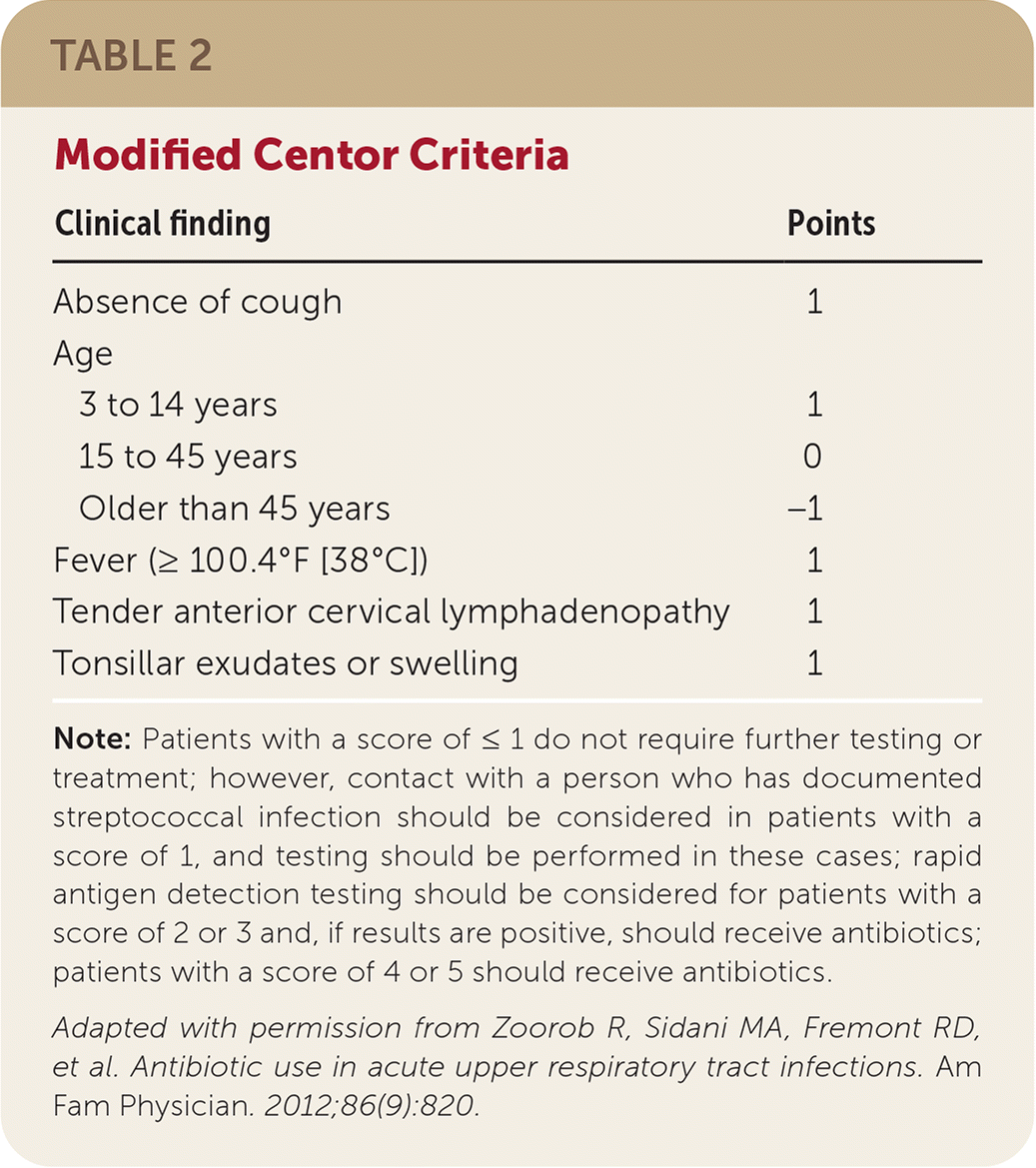
| Clinical finding | Points |
|---|---|
| Absence of cough | 1 |
| Age | |
| 3 to 14 years | 1 |
| 15 to 45 years | 0 |
| Older than 45 years | −1 |
| Fever (≥ 100.4°F [38°C]) | 1 |
| Tender anterior cervical lymphadenopathy | 1 |
| Tonsillar exudates or swelling | 1 |
The Infectious Diseases Society of America recommends that patients with suspected group A beta-hemolytic streptococcal pharyngitis undergo a throat swab rapid antigen detection test (RADT) to aid in diagnosis. Positive RADT results are sufficient for the diagnosis and treatment of group A beta-hemolytic streptococcal pharyngitis due to the test’s high specificity.13 Throat culture should be obtained in children and adolescents with negative RADT results because of the high prevalence of group A beta-hemolytic streptococcal infection in this age group and lower sensitivity of RADTs.13 In contrast, adults with negative RADT results do not require a confirmatory throat culture because the incidence of group A beta-hemolytic streptococcal pharyngitis and acute rheumatic fever are low in adults.13,37 Point-of-care molecular testing for the diagnosis of group A beta-hemolytic streptococcal pharyngitis is also available and is a more sensitive option than RADTs and bacterial culture with a fast turnaround time. However, molecular testing is more expensive than RADTs and bacterial cultures, and patients who are colonized with group A beta-hemolytic streptococcus can erroneously be diagnosed with group A beta-hemolytic streptococcal pharyngitis when their symptoms are due to another pathogen. Patients with other bacterial causes of pharyngitis will be missed on molecular testing.38 Testing for group A beta-hemolytic streptococcal pharyngitis is discouraged in children and adults who have signs or symptoms suggestive of a viral etiology such as diarrhea, rhinorrhea, or cough, and in children younger than three years because of the low incidence of streptococcal pharyngitis and acute rheumatic fever in this age group.13,39 A 10-day course of penicillin V or amoxicillin is the first-line therapy for group A beta-hemolytic streptococcal pharyngitis.13 Analgesic or antipyretic medications should also be considered.
Epiglottitis
Epiglottitis is a rare, life-threatening condition resulting from inflammatory edema of the epiglottis and surrounding supraglottic tissues, often due to infection. Haemophilus influenzae type b (Hib) is no longer the most common cause of epiglottitis since the vaccine was developed in 1985, and Hib now affects adults more than children.15,40 Streptococcus pneumoniae, group A beta-hemolytic streptococcus, and Staphylococcus aureus are among the pathogens responsible for epiglottitis; however, Hib continues to account for some cases due to insufficient herd immunity and vaccine failure.16,40 Children with epiglottitis usually present with the rapid development of a high fever, inspiratory stridor, restlessness, drooling, a muffled “hot potato” voice, and a preference to sit forward in the “sniffing” position. Adults generally present with slower onset of sore throat, odynophagia, dysphagia, fever, dyspnea, hoarseness, muffled voice, drooling, cough, and stridor.15 Epiglottitis is diagnosed through direct visualization with laryngoscopy or radiography demonstrating an enlarged epiglottis. Treatment includes broad-spectrum intravenous antibiotics such as third-generation cephalosporins or ampicillin/sulbactam (Unasyn) and may also require methicillin-resistant S. aureus or other bacterial or fungal coverage.15–17
Rhinosinusitis
Acute rhinosinusitis is inflammation of the nasal cavities and paranasal sinuses of less than four weeks’ duration that is typically diagnosed using clinical criteria. Common presenting symptoms include fever, purulent nasal discharge, facial pain, and headache. Viruses are responsible for 90% to 98% of acute rhinosinusitis cases.41 Comparatively, acute bacterial rhinosinusitis develops in 0.5% to 2% of all URIs, with only a small percentage of these cases warranting antibiotics.42
A Cochrane review of treatments for acute rhinosinusitis concluded that considering antibiotic resistance and the low incidence of serious complications, antibiotics are not recommended to treat uncomplicated acute rhinosinusitis.43 Data support the use of antibiotics in some cases of acute bacterial rhinosinusitis. Because no consensus on the diagnostic criteria has been established, physicians must rely on existing guidelines to determine when to start antibiotics. According to the Infectious Diseases Society of America guidelines, antibiotics should be started for patients manifesting any one of the constellations of symptoms outlined in Table 3.9

| Treat acute bacterial rhinosinusitis in patients with any one of the following symptom complexes: |
| Onset with severe symptoms or signs, including high fever (≥ 102.2°F [39°C]) and purulent nasal discharge or facial pain lasting for at least three to four consecutive days at the beginning of the illness |
| Persistent symptoms or signs compatible with acute bacterial rhinosinusitis lasting ≥ 10 days without clinical improvement |
| Worsening symptoms or signs for three to four days characterized by the onset of fever, headache, or increase in nasal discharge following a typical viral upper respiratory tract infection that lasted five to six days and was initially improving (i.e., double sickening) |
The predominant causes of acute bacterial rhinosinusitis are H. influenzae, S. pneumoniae, and Moraxella catarrhalis. Based on this microbiology, the first-line treatment for acute bacterial rhinosinusitis is amoxicillin/clavulanate (Augmentin), given for five to seven days in adults and 10 to 14 days in children.9,10
Interventions to Reduce Unnecessary Antibiotic Use in Acute URIs
Antibiotic resistance is among the greatest public health threats today. The CDC estimates that more than 2 million antibiotic-resistant infections result in at least 23,000 deaths annually in the United States.44 Antibiotic stewardship is a national effort to improve antibiotic prescribing by clinicians and use by patients so that antibiotics are only prescribed and used when needed. Antibiotic stewardship also ensures that the right medication, dose, and duration are selected. Because penicillins are the first-line treatments for URIs requiring antibiotics, identifying patients with severe penicillin allergies for whom penicillins and possibly cephalosporins should be avoided is an essential part of antibiotic stewardship. Approximately 10% of people in the United States report having a penicillin allergy; however, less than 1% of the population has a confirmed penicillin allergy.45 Cephalosporins are often avoided in patients who are allergic to penicillin for fear of cross-reactivity; however, data suggest this risk is low and many cephalosporins can be safely used in patients who report a penicillin allergy.46 PEN-FAST (penicillin allergy, five or fewer years ago, anaphylaxis/angioedema, severe cutaneous adverse reaction, and treatment required for allergy episode) is a validated, point-of-care clinical decision rule to aid physicians in their selection of antibiotics for patients who report a history of a penicillin allergy.47
Evaluating the effectiveness of interventions to reduce antibiotic prescriptions is important because inappropriate antibiotic use is one of the most modifiable risk factors for antibiotic resistance. The CDC’s review of antibiotic stewardship identified high-priority conditions to target for improving antibiotic prescribing, including nonspecific URI, viral pharyngitis, uncomplicated acute bacterial rhinosinusitis, and AOM. A review of the Agency for Healthcare Research and Quality summary on the effectiveness and adverse consequences of strategies to reduce antibiotic use in adults and children with uncomplicated URIs found that RADT in adults reduced inappropriate antibiotic prescribing without adverse consequences. Delayed prescribing reduced antibiotic use but also decreased patient satisfaction and increased symptom duration.48 A similar review of 133 studies found the best evidence for three URI interventions that improved or reduced antibiotic prescribing without causing significant adverse consequences. These interventions included clinic-based parent education, public patient education campaigns combined with clinician education, and electronic decision support systems.49
A cross-sectional study of 1,285 children’s visits for acute URIs between December 2007 and April 2009 demonstrated that a combination of positive treatment recommendations (i.e., suggestions to reduce a patient’s symptoms) with negative treatment recommendations (i.e., explanations of the inappropriateness of antibiotics) was associated with decreased antibiotic prescribing and higher visit satisfaction ratings.50 In a 2021 review of antibiotic use in the United States and the core elements of outpatient antibiotic stewardship, the CDC has provided the results of a systematic review of interventions and outcomes supporting outpatient antibiotic stewardship. A summary of the CDC review of interventions with a positive outcome can be found in Table 4.9,11,13,51,52 Implementation of these strategies and adherence to antibiotic stewardship in the treatment of URIs decrease antibiotic resistance, adverse patient outcomes, and unnecessary health care costs. Table 5 lists physician and patient resources for antibiotic stewardship.
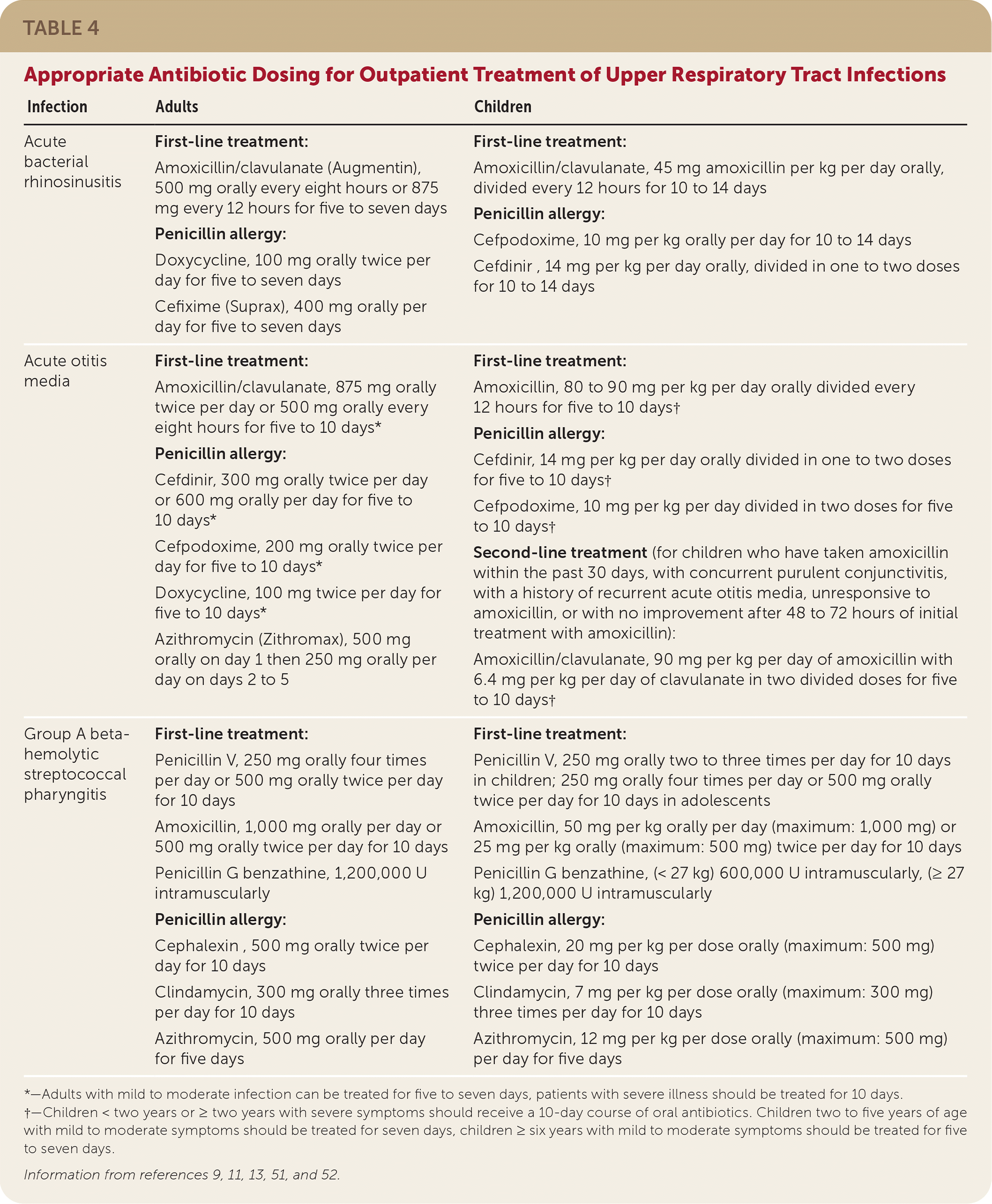
| Infection | Adults | Children |
|---|---|---|
| Acute bacterial rhinosinusitis | First-line treatment: Amoxicillin/clavulanate (Augmentin), 500 mg orally every eight hours or 875 mg every 12 hours for five to seven days Penicillin allergy: Doxycycline, 100 mg orally twice per day for five to seven days Cefixime (Suprax), 400 mg orally per day for five to seven days | First-line treatment: Amoxicillin/clavulanate, 45 mg amoxicillin per kg per day orally, divided every 12 hours for 10 to 14 days Penicillin allergy: Cefpodoxime, 10 mg per kg orally per day for 10 to 14 days Cefdinir , 14 mg per kg per day orally, divided in one to two doses for 10 to 14 days |
| Acute otitis media | First-line treatment: Amoxicillin/clavulanate, 875 mg orally twice per day or 500 mg orally every eight hours for five to 10 days* Penicillin allergy: Cefdinir, 300 mg orally twice per day or 600 mg orally per day for five to 10 days* Cefpodoxime, 200 mg orally twice per day for five to 10 days* Doxycycline, 100 mg twice per day for five to 10 days* Azithromycin (Zithromax), 500 mg orally on day 1 then 250 mg orally per day on days 2 to 5 | First-line treatment: Amoxicillin, 80 to 90 mg per kg per day orally divided every 12 hours for five to 10 days† Penicillin allergy: Cefdinir, 14 mg per kg per day orally divided in one to two doses for five to 10 days† Cefpodoxime, 10 mg per kg per day divided in two doses for five to 10 days† Second-line treatment (for children who have taken amoxicillin within the past 30 days, with concurrent purulent conjunctivitis, with a history of recurrent acute otitis media, unresponsive to amoxicillin, or with no improvement after 48 to 72 hours of initial treatment with amoxicillin): Amoxicillin/clavulanate, 90 mg per kg per day of amoxicillin with 6.4 mg per kg per day of clavulanate in two divided doses for five to 10 days† |
| Group A beta-hemolytic streptococcal pharyngitis | First-line treatment: Penicillin V, 250 mg orally four times per day or 500 mg orally twice per day for 10 days Amoxicillin, 1,000 mg orally per day or 500 mg orally twice per day for 10 days Penicillin G benzathine, 1,200,000 U intramuscularly Penicillin allergy: Cephalexin , 500 mg orally twice per day for 10 days Clindamycin, 300 mg orally three times per day for 10 days Azithromycin, 500 mg orally per day for five days | First-line treatment: Penicillin V, 250 mg orally two to three times per day for 10 days in children; 250 mg orally four times per day or 500 mg orally twice per day for 10 days in adolescents Amoxicillin, 50 mg per kg orally per day (maximum: 1,000 mg) or 25 mg per kg orally (maximum: 500 mg) twice per day for 10 days Penicillin G benzathine, (< 27 kg) 600,000 U intramuscularly, (≥ 27 kg) 1,200,000 U intramuscularly Penicillin allergy: Cephalexin, 20 mg per kg per dose orally (maximum: 500 mg) twice per day for 10 days Clindamycin, 7 mg per kg per dose orally (maximum: 300 mg) three times per day for 10 days Azithromycin, 12 mg per kg per dose orally (maximum: 500 mg) per day for five days |
| American Heart Association Physician Alliance | http://www.ahaphysicianforum.org/resources/appropriate-use/antimicrobial/index.shtml |
| Centers for Disease Control and Prevention | https://www.cdc.gov/antibiotic-use |
| Choosing Wisely Canada | https://choosingwiselycanada.org/primary-care/antibiotics |
| Interactive Medical Training Resources | https://www.uwimtr.org/dart |
This article updates previous articles on this topic by Wong, et al.,53 and Zoorob, et al.35
Data Sources: A search was completed in PubMed using the key terms antibiotics, upper respiratory infections, diagnosis, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, the Cochrane database, Essential Evidence Plus, and Choosing Wisely. Search dates: September 9 to November 22, 2021, and August 31, 2022.