
Am Fam Physician. 2023;107(2):173-181
Related editorial: Comparing Clinical Guidelines for the Management of Major Depressive Disorder
Patient information: See related handout on treating depression with medicine.
Author disclosure: No relevant financial relationships.
The prevalence of depression and the use of antidepressant medications have risen steadily in the United States over the past three decades. Antidepressants are the most commonly prescribed medications for U.S. adults 20 to 59 years of age. Second-generation antidepressants (e.g., selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, serotonin modulators, atypical antidepressants) are first-line therapy for depression. Psychotherapy, including cognitive behavior therapy and other types of individual and group therapy, is also a first-line treatment. The combination of medication and psychotherapy is preferred for severe depression. Treatment history, comorbidities, costs, and risk of adverse effects should be considered when choosing an antidepressant medication. Although many patients use antidepressants indefinitely, few studies have examined safety and effectiveness beyond two years. There is an increased risk of relapse or recurrence of depressive symptoms when an antidepressant is discontinued, compared with continued use. Gradually tapering the dosage while concurrently providing cognitive behavior therapy can decrease this risk. High-quality evidence on antidepressant use in pregnancy is lacking. Depression and use of antidepressants are both associated with preterm birth.
The use of antidepressant medications in the United States has increased fivefold since the introduction of selective serotonin reuptake inhibitors (SSRIs) in the late 1980s.1,2 Between 2015 and 2018, the percentage of U.S. adults who reported taking an antidepressant medication in the past 30 days was 13.2%, compared with 2.4% between 1988 and 1994.1,2
| Between 2015 and 2018, the percentage of U.S. adults who reported taking an antidepressant medication in the past 30 days was 13.2%, compared with 2.4% between 1988 and 1994. |
| Modest evidence shows that escitalopram, mirtazapine, paroxetine, venlafaxine, and amitriptyline are the most effective antidepressants for reducing acute depressive symptoms by greater than 50% at eight weeks. |
| A 2021 network meta-analysis demonstrated a low risk of ventricular arrhythmia or sudden cardiac death in those taking selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, or tricyclic antidepressants. |
| Typical symptoms of antidepressant discontinuation syndrome can be described using the FINISH mnemonic (flulike symptoms, insomnia, nausea, imbalance, sensory disturbances, hyperarousal). |
Antidepressants are the most commonly prescribed medications for U.S. adults 20 to 59 years of age.3 Rates of depression and suicide have increased, primarily among those younger than 25 years.4,5 The percentage of adults 18 to 25 years of age reporting a major depressive episode in the past year doubled from 8.8% in 2005 to 17% in 2020. During the same period, rates among adults 26 years and older increased only slightly from 6.2% to 7.1%.6,7
The definition of a major depressive episode is based on the Diagnostic and Statistical Manual of Mental Disorders, 5th ed., criteria for major depressive disorder.6,8 Five or more depressive symptoms must be present for at least two weeks, cause distress or functional impairment, and not be due to another medical or psychiatric condition. Symptoms include depressed mood, anhedonia, changes in weight or sleep patterns, fatigue, psychomotor agitation or retardation, feelings of worthlessness or guilt, impaired concentration, and recurrent thoughts of death.6,8 Clinical trials of antidepressants often use major depressive episode as an inclusion criterion. Although most patients with clinician-identified depression do not meet diagnostic criteria for a major depressive episode, many are prescribed antidepressants.9
Second-generation antidepressants are the most common medications used to treat depression in the United States.10 These include SSRIs (e.g., escitalopram, paroxetine), serotonin-norepinephrine reuptake inhibitors (SNRIs; e.g., duloxetine [Cymbalta], venlafaxine), serotonin modulators (e.g., nefazodone, trazodone), and atypical antidepressants (e.g., bupropion, mirtazapine).
Effectiveness
Despite thousands of clinical trials, the effectiveness of antidepressants is not well established. High-quality reviews of randomized controlled studies show a statistically significant improvement in depression with use of antidepressant medications.11,12 A 2016 systematic review showed that the number needed to treat for response to treatment or remission is 9 for tricyclic antidepressants, 7 for SSRIs, and 6 for venlafaxine.11 Outcomes of other studies challenge these conclusions, with minimal difference in symptoms between placebo and antidepressants, publication bias favoring effectiveness, and pharmaceutical industry sponsorship of most clinical trials.11,13,14 A recent national survey of adults with depression revealed that those who used antidepressants had no improvement in health-related quality of life at two years of follow-up compared with those who did not use antidepressants.15
Psychotherapy (e.g., behavior therapy, cognitive therapy, cognitive behavior therapy, interpersonal psychotherapy, psychodynamic therapy, supportive therapy) is also a first-line treatment for depression.16,17 The effectiveness of psychotherapy is similar to that of antidepressants in the primary care setting (relative risk [RR] = 1.03; 95% CI, 0.88 to 1.22).18 Evidence for cognitive behavior therapy is more robust than for other types of therapy.10 The combination of psychotherapy and pharmacotherapy may be more effective than either treatment alone for moderate or severe depression and may reduce risk of relapse and recurrence.17,19,20
Selection of Initial Depression Therapy
Guidelines from the United Kingdom's National Institute for Health and Care Excellence recommend against routinely offering medication for mild to moderate depression (defined as a Patient Health Questionnaire-9 score of less than 16). If the patient prefers medication, SSRIs are recommended. Active monitoring, group exercise, and several types of individual and group therapy are recommended as management options. For more severe depression, a combination of individual cognitive behavior therapy and an antidepressant (SSRI or SNRI) is recommended.17
Shared decision-making should be used when choosing an initial treatment. Prior treatment and response, comorbidities, costs, and risk of adverse effects should be considered. Pharmacogenetic testing, intended to tailor therapy to an individual's genetic variants, has not shown consistent benefit in outcomes or cost-effectiveness.21
For the general adult population, treatment should start with a second-generation antidepressant or psychotherapy.10,16,17 If an antidepressant is selected, modest evidence shows that escitalopram, mirtazapine, paroxetine, venlafaxine, and amitriptyline are most effective in reducing depressive symptoms by greater than 50% at eight weeks (odds ratio = 1.19 to 1.96).12
Adverse Effects
Nausea and vomiting were the most commonly reported symptoms leading to antidepressant discontinuation during clinical trials. Patients taking SNRIs have a higher incidence of nausea and vomiting than those taking SSRIs.22 Other common adverse effects across drug classes include sexual adverse effects and weight gain, although these are less likely with bupropion.22,23
Despite concerns about increased risk of cardiac arrhythmia with SSRIs, a 2021 network meta-analysis demonstrated a low risk of ventricular arrhythmia or sudden cardiac death in those taking SSRIs, SNRIs, or tricyclic antidepressants.24 QT prolongation can occur with these drug classes; therefore, caution should be used when combining them with other medications that cause QT prolongation (e.g., antiemetics, antiarrhythmics, neuroleptics). Bupropion has the lowest risk of QT prolongation in patients at high risk of ventricular arrhythmias. Because of its side effect profile and potential for beneficial antiplatelet activity, sertraline may be preferred for those with ischemic heart disease.25
Long-term adverse effects should also be considered when prescribing antidepressants. Limited-quality evidence, including a 12-year cohort study, found a correlation between SSRI use and falls (hazard ratio [HR] = 1.48; 95% CI, 1.39 to 1.59); fractures (HR = 1.30; 95% CI, 1.21 to 1.39); and all-cause mortality (HR = 1.38; 95% CI, 1.26 to 1.51).26
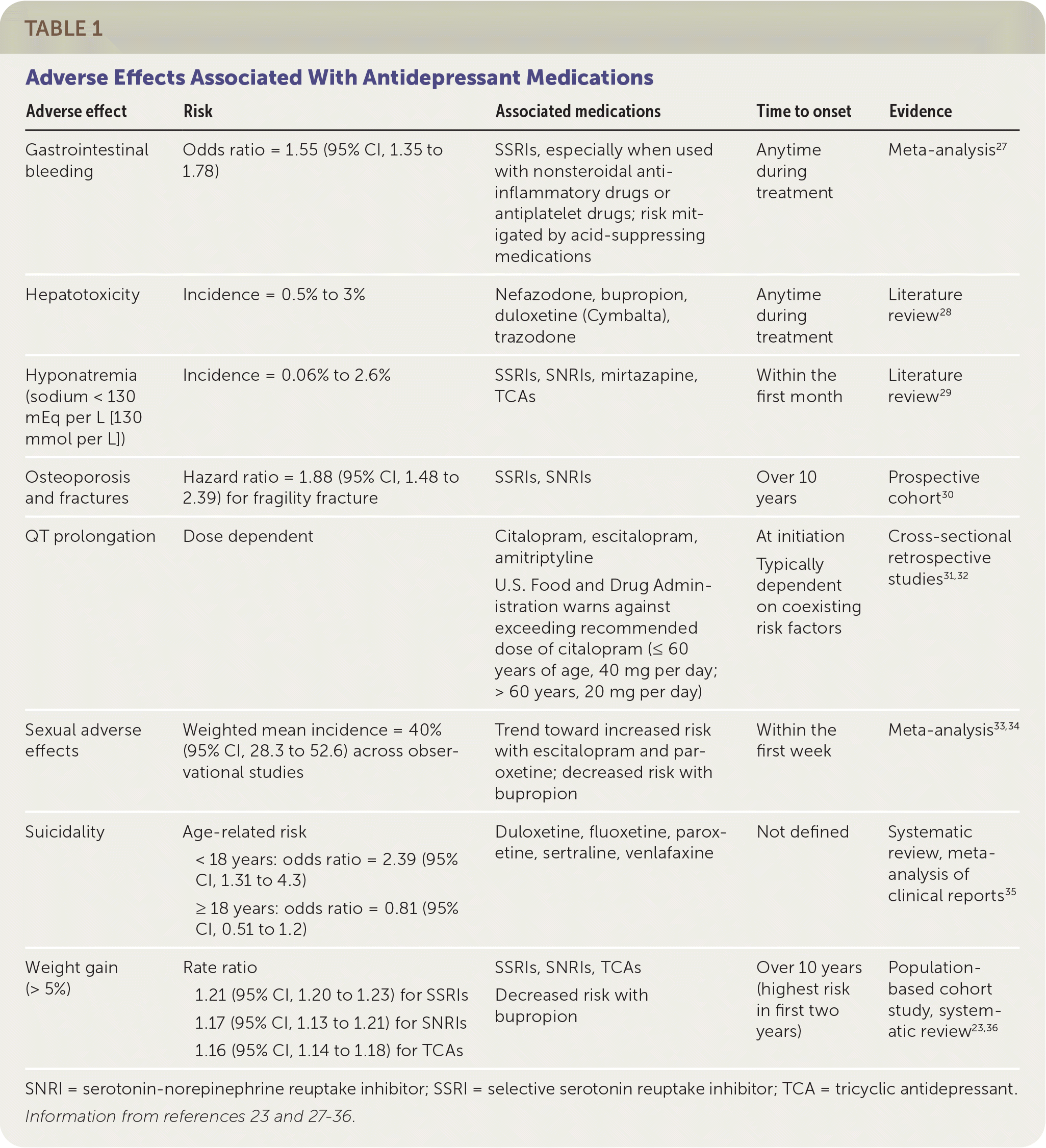
| Adverse effect | Risk | Associated medications | Time to onset | Evidence |
|---|---|---|---|---|
| Gastrointestinal bleeding | Odds ratio = 1.55 (95% CI, 1.35 to 1.78) | SSRIs, especially when used with nonsteroidal anti-inflammatory drugs or antiplatelet drugs; risk mitigated by acid-suppressing medications | Anytime during treatment | Meta-analysis27 |
| Hepatotoxicity | Incidence = 0.5% to 3% | Nefazodone, bupropion, duloxetine (Cymbalta), trazodone | Anytime during treatment | Literature review 28 |
| Hyponatremia (sodium < 130 mEq per L [130 mmol per L]) | Incidence = 0.06% to 2.6% | SSRIs, SNRIs, mirtazapine, TCAs | Within the first month | Literature review 29 |
| Osteoporosis and fractures | Hazard ratio = 1.88 (95% CI, 1.48 to 2.39) for fragility fracture | SSRIs, SNRIs | Over 10 years | Prospective cohort30 |
| QT prolongation | Dose dependent | Citalopram, escitalopram, amitriptyline U.S. Food and Drug Administration warns against exceeding recommended dose of citalopram (≤ 60 years of age, 40 mg per day; > 60 years, 20 mg per day) | At initiation Typically dependent on coexisting risk factors | Cross-sectional retrospective studies31,32 |
| Sexual adverse effects | Weighted mean incidence = 40% (95% CI, 28.3 to 52.6) across observational studies | Trend toward increased risk with escitalopram and paroxetine; decreased risk with bupropion | Within the first week | Meta-analysis33,34 |
| Suicidality | Age-related risk < 18 years: odds ratio = 2.39 (95% CI, 1.31 to 4.3) ≥ 18 years: odds ratio = 0.81 (95% CI, 0.51 to 1.2) | Duloxetine, fluoxetine, paroxetine, sertraline, venlafaxine | Not defined | Systematic review, meta-analysis of clinical reports35 |
| Weight gain (> 5%) | Rate ratio 1.21 (95% CI, 1.20 to 1.23) for SSRIs 1.17 (95% CI, 1.13 to 1.21) for SNRIs 1.16 (95% CI, 1.14 to 1.18) for TCAs | SSRIs, SNRIs, TCAs Decreased risk with bupropion | Over 10 years (highest risk in first two years) | Population-based cohort study, systematic review23,36 |
Duration of Treatment
The treatment of depression is often described in three phases. The acute phase of six to 12 weeks is intended to induce remission of symptoms and aid in recovery of function. The continuation phase of four to nine months is aimed at reducing relapse (return of symptoms). The maintenance phase is intended to prevent recurrence (a new episode of depression) after one year of treatment.10,37
Although up to 75% of patients discontinue antidepressant use within six months, others continue indefinitely.38 The increase in antidepressant use over the past 30 years is largely due to longer treatment duration.39 The longer patients are in the maintenance phase, the less often treatment is reviewed by their primary care physicians.40 Few studies have evaluated safety and effectiveness beyond two years.41
U.S. guidelines do not specify a duration of treatment for antidepressants.10,16 Canadian guidelines recommend at least six months of treatment and two years or more for those at higher risk of relapse.45 Because each episode of depression adds to a patient's risk of future episodes, indefinite maintenance treatment is often recommended for patients with three or more episodes of depression.37
Discontinuation
The Diagnostic and Statistical Manual of Mental Disorders, 5th ed., defines antidepressant discontinuation syndrome as “a set of symptoms that can occur after an abrupt cessation (or marked reduction in dose) of an antidepressant medication that was taken continuously for at least one month.”8 Effects typically manifest within two to four days and can last for several months.46 Typical symptoms can be described using the FINISH mnemonic (flulike symptoms, insomnia, nausea, imbalance, sensory disturbances, hyperarousal).47 Patients should be counseled on symptoms that may occur with abrupt cessation of treatment.48
Symptoms of antidepressant discontinuation syndrome can be difficult to distinguish from relapse and recurrence. The prevalence of these symptoms varies, but some reviews have found that 50% of patients are affected.46 Regardless of pharmacologic mechanism, the risk of antidepressant discontinuation syndrome is higher for drugs with a shorter half-life (Table 2).49,50

| Antidepressant | Risk* |
|---|---|
| Atypical antidepressant | |
| Bupropion | + |
| Mirtazapine | + |
| Serotonin-norepinephrine reuptake inhibitor | |
| Desvenlafaxine (Pristiq) | +++ |
| Venlafaxine | +++ |
| Duloxetine (Cymbalta) | ++ |
| Milnacipran | + |
| SSRI | |
| Fluvoxamine | +++ |
| Paroxetine | +++ |
| Citalopram | ++ |
| Escitalopram | ++ |
| Sertraline | ++ |
| Fluoxetine | + |
| SSRI/partial 5-HT1Areceptor agonist | |
| Trazodone | ++ |
| Vortioxetine (Trintellix) | ++ |
| Vilazodone (Viibryd) | + |
| Tricyclic antidepressant | |
| Imipramine | +++ |
| Nortriptyline | +++ |
| Amitriptyline | ++ |
| Clomipramine | ++ |
| Desipramine | ++ |
| Doxepin | ++ |
Despite a lack of head-to-head trials, research shows that a slow medication taper of at least 14 days is best practice; a taper of several months may be needed.51–54 Tapering strategies are detailed in Table 3.51–53 A dose taper of approximately 25% every four weeks and a faster taper of 12.5% every two weeks are both reasonable strategies. A gradual taper has been shown to result in as few as 5% of patients experiencing discontinuation symptoms.55 Use of cognitive behavior therapy during the medication taper may help prevent relapse or recurrence.56
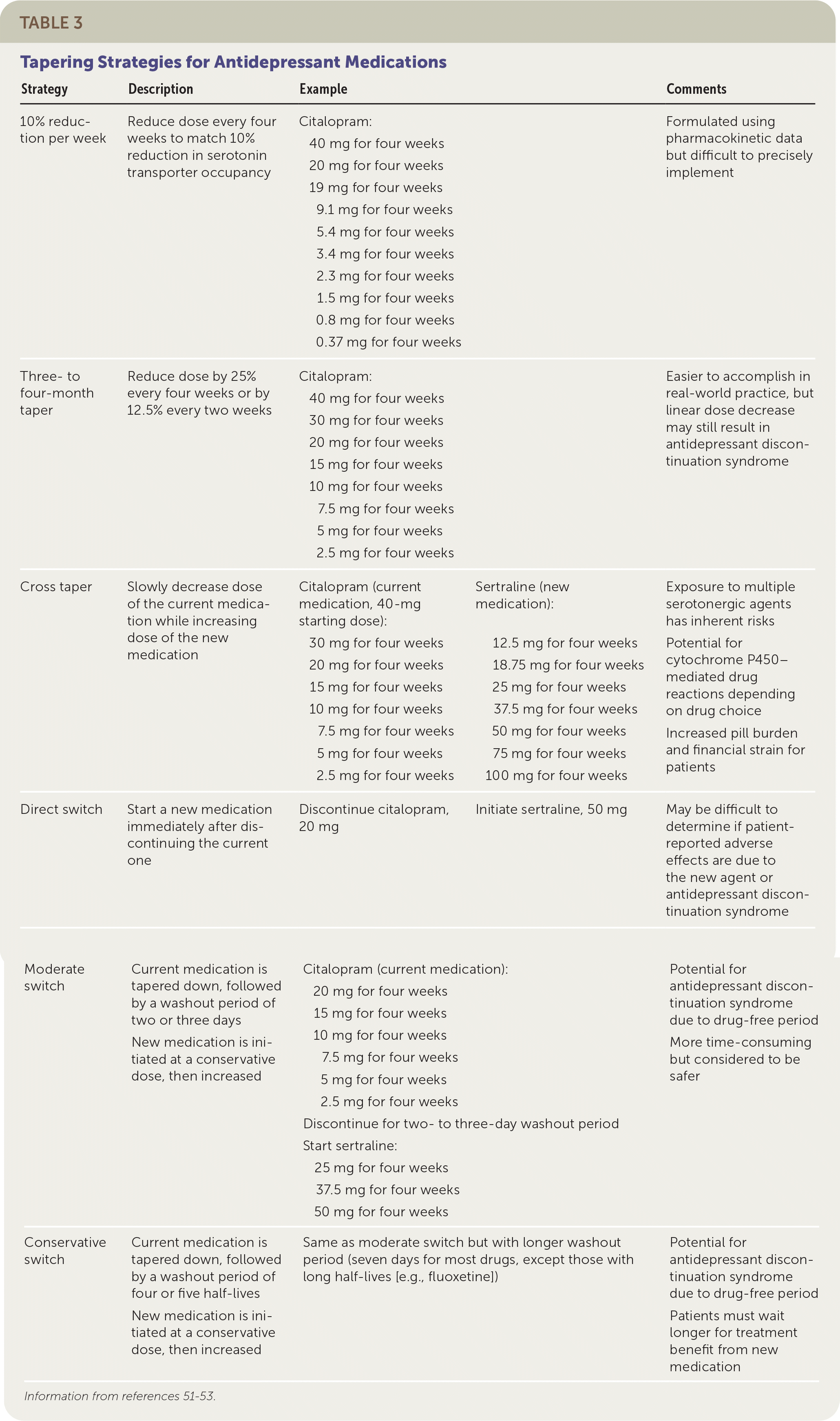
| Strategy | Description | Example | Comments | ||
|---|---|---|---|---|---|
| 10% reduction per week | Reduce dose every four weeks to match 10% reduction in serotonin transporter occupancy | Citalopram: 40 mg for four weeks 20 mg for four weeks 19 mg for four weeks 9.1 mg for four weeks 5.4 mg for four weeks 3.4 mg for four weeks 2.3 mg for four weeks 1.5 mg for four weeks 0.8 mg for four weeks 0.37 mg for four weeks | Formulated using pharmacokinetic data but difficult to precisely implement | ||
| Three- to four-month taper | Reduce dose by 25% every four weeks or by 12.5% every two weeks | Citalopram: 40 mg for four weeks 30 mg for four weeks 20 mg for four weeks 15 mg for four weeks 10 mg for four weeks 7.5 mg for four weeks 5 mg for four weeks 2.5 mg for four weeks | Easier to accomplish in real-world practice, but linear dose decrease may still result in antidepressant discontinuation syndrome | ||
| Cross taper | Slowly decrease dose of the current medication while increasing dose of the new medication | Citalopram (current medication, 40-mg starting dose): 30 mg for four weeks 20 mg for four weeks 15 mg for four weeks 10 mg for four weeks 7.5 mg for four weeks 5 mg for four weeks 2.5 mg for four weeks | Sertraline (new medication): 12.5 mg for four weeks 18.75 mg for four weeks 25 mg for four weeks 37.5 mg for four weeks 50 mg for four weeks 75 mg for four weeks 100 mg for four weeks | Exposure to multiple serotonergic agents has inherent risks Potential for cytochrome P450– mediated drug reactions depending on drug choice Increased pill burden and financial strain for patients | |
| Direct switch | Start a new medication immediately after discontinuing the current one | Discontinue citalopram, 20 mg | Initiate sertraline, 50 mg | May be difficult to determine if patient-reported adverse effects are due to the new agent or antidepressant discontinuation syndrome | |
| Moderate switch | Current medication is tapered down, followed by a washout period of two or three days New medication is initiated at a conservative dose, then increased | Citalopram (current medication): 20 mg for four weeks 15 mg for four weeks 10 mg for four weeks 7.5 mg for four weeks 5 mg for four weeks 2.5 mg for four weeks Discontinue for two- to three-day washout period Start sertraline: 25 mg for four weeks 37.5 mg for four weeks 50 mg for four weeks | Potential for antidepressant discontinuation syndrome due to drug-free period More time-consuming but considered to be safer | ||
| Conservative switch | Current medication is tapered down, followed by a washout period of four or five half-lives New medication is initiated at a conservative dose, then increased | Same as moderate switch but with longer washout period (seven days for most drugs, except those with long half-lives [e.g., fluoxetine]) | Potential for antidepressant discontinuation syndrome due to drug-free period Patients must wait longer for treatment benefit from new medication | ||
Special Populations
PREGNANT PATIENTS
Approximately 12% of patients in the perinatal period meet criteria for major depressive disorder.57 Patients with untreated depression during pregnancy have a higher incidence of preterm birth and low-birth-weight infants compared with those without depression.58 Treatment of depression has not been shown to improve these outcomes, and SSRIs may be independently associated with preterm birth.59,60
Screening pregnant and postpartum patients for depression is associated with a 2% to 9% reduction in absolute risk of depression at three to five months, with or without treatment.61 The U.S. Preventive Services Task Force recommends that clinicians provide or refer pregnant and postpartum patients who are at increased risk of perinatal depression to counseling interventions.62
For patients taking antidepressants before pregnancy, discontinuation is more likely to lead to relapse when depression is severe or recurrent. A meta-analysis showed that discontinuation of antidepressants in patients with mild to moderate depression is not significantly associated with relapse.63 Patients should continue their antidepressant when, through shared decision-making, the risk of relapse is determined to be greater than the risk of rare neonatal complications.
Cohort studies have inconsistently shown a small correlation between first-trimester SSRI use and cardiac malformations (RR = 1.24; 95% CI, 1.11 to 1.37).64–66 SSRI use during the third trimester may increase the risk of newborn respiratory distress, tremors, and admission to the neonatal intensive care unit.67–69 Discontinuation of SSRIs in the third trimester does not improve these outcomes.70 There are no data on long-term neurocognitive effects.
A U.S. Food and Drug Administration advisory on SSRI use during pregnancy and the risk of persistent pulmonary hypertension in newborns cites conflicting findings; a causal relationship is unclear.71 A systematic review and meta-analysis found a slightly increased risk of persistent pulmonary hypertension in newborns with prenatal exposure to SSRIs and SNRIs (number needed to harm = 1,000).72
BREASTFEEDING PATIENTS
Antidepressants transfer into breast milk in low concentrations. This transfer is thought to be lower for paroxetine and sertraline than other antidepressants, producing undetectable concentrations in infant plasma. Fluoxetine and venlafaxine produce the highest infant plasma concentrations. Potential adverse effects in infants exposed to SSRIs via breast milk have been documented only in case reports, most commonly with fluoxetine and citalopram. The effects are nonspecific and include irritability and decreased feeding. Overall, there is little evidence to support a causal link between antidepressant use in breastfeeding patients and adverse effects in their infants.73
OLDER ADULTS
Approximately 50% of patients older than 65 years who have depression report at least a 50% improvement in symptoms with antidepressant use.74 Although prior studies showed no difference in the effectiveness of antidepressants in older patients, a 2019 network meta-analysis found that response rates are significantly higher with quetiapine (RR = 2.09) and duloxetine (RR = 1.83) in this population compared with placebo.75 Quetiapine includes a U.S. Food and Drug Administration boxed warning due to increased mortality risk in older patients with dementia-related psychosis. Some drugs are associated with higher rates of remission compared with placebo: quetiapine (RR = 2.38), mirtazapine (RR = 1.90), and duloxetine (RR = 1.52). In older patients, fall risk should be evaluated and steps taken to mitigate the risk because untreated depression and antidepressant use can both contribute to falls.76
Because older adults are at greater risk of adverse drug reactions, initiating treatment at approximately one-half of the usual adult starting dose is often recommended. Guidelines recommend sertraline, duloxetine, or escitalopram as good first-line options for older patients.77 Bupropion, mirtazapine, and venlafaxine are also considered appropriate because of their favorable side effect profiles. Paroxetine is associated with more anticholinergic effects, and fluoxetine has a greater risk of agitation and overstimulation; neither should be used in older adults.77 Before initiating SSRIs and SNRIs in older adults, clinicians should screen for a history of hyponatremia and measure serum sodium level two to four weeks after initiating therapy.77
This article updates previous articles on this topic by Kovich and DeJong78; Adams, et al.79; and Warner, et al.80
Data Sources: PubMed searches were completed using key terms such as depression, antidepressant, and antidepressant discontinuation and specific classes of antidepressant medications (e.g., selective serotonin reuptake inhibitor). Additional terms were added to further refine results. For example, after an initial search generated a list of common antidepressant adverse effects, each effect was searched separately. The search included meta-analyses, randomized controlled trials, systematic reviews, and clinical trials. The U.S. Preventive Services Task Force recommendations were referenced, and citations from relevant recommendations were examined. Essential Evidence Plus and the Cochrane database were also searched. Search dates: February through December 2022.
