
Am Fam Physician. 2023;107(5):online
Author disclosure: No relevant financial relationships.

Details for This Review
Study Population: 1,818 adults with alcohol use disorder in 17 randomized controlled trials; the mean age was 46.5 years, and 70% were men
Efficacy End Points: Relapse, frequency of alcohol use, amount of alcohol use
Harm End Points: Adverse events
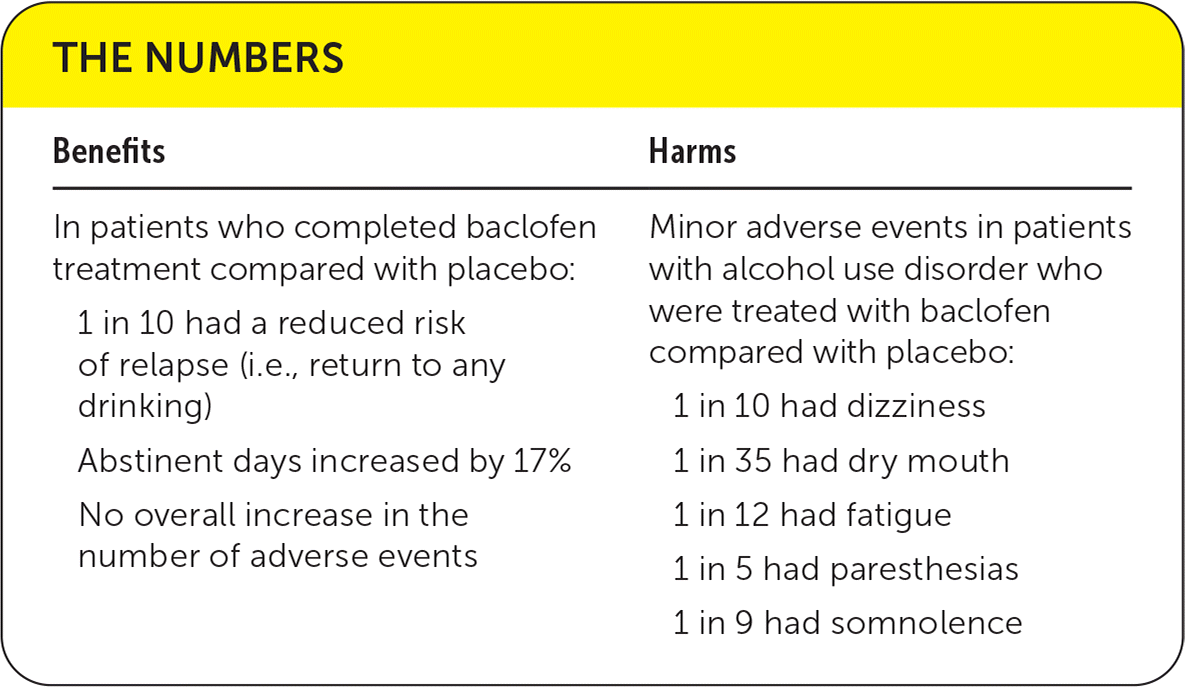
| Benefits | Harms |
|---|---|
| In patients who completed baclofen treatment compared with placebo: 1 in 10 had a reduced risk of relapse (i.e., return to any drinking) Abstinent days increased by 17% No overall increase in the number of adverse events | Minor adverse events in patients with alcohol use disorder who were treated with baclofen compared with placebo: 1 in 10 had dizziness 1 in 35 had dry mouth 1 in 12 had fatigue 1 in 5 had paresthesias 1 in 9 had somnolence |
Narrative: Alcohol use disorder is characterized by the inability to control alcohol consumption and frequent drinking episodes leading to clinically significant impairment or distress.1,2 In U.S. adults, alcohol use disorder has a 12-month prevalence of 13.9% and a lifetime prevalence of 29.1%.3 Alcohol use disorder increases the risk of cancer, liver disease, cardiac disease, unintentional accidents, and neurologic conditions.4 Treatment options supported by the American Psychiatric Association include individualized plans that focus on nonpharmacologic approaches (including cognitive behavior therapy and 12-step therapies such as Alcoholics Anonymous) and pharmacotherapy. Pharmacologic options include opioid antagonists (naltrexone, nalmefene), glutamate antagonists (acamprosate), aversive agents (disulfiram), and anticonvulsants (gabapentin, topiramate).5
Baclofen is a selective agonist of gamma-aminobutyric acid receptors that was initially approved for the treatment of muscle spasticity and is now being evaluated for the treatment of alcohol use disorder.6,7 Baclofen's potential mechanism of action is through suppression of alcohol-stimulated dopamine release.6
The Cochrane review included 17 randomized controlled trials that evaluated the benefits and risks of baclofen for the treatment of alcohol use disorder, with primary outcomes of relapse, frequency of use, amount of use, and adverse events.8 Secondary outcomes included cravings, anxiety, depression, and other known adverse events. Overall, there was a low risk of bias in the included studies. The randomized controlled trials had at least a 12-week overall duration and a four-week minimum treatment course, with doses of baclofen between 30 mg and 300 mg.
The Cochrane review showed that baclofen reduces the risk of relapse compared with placebo at the end of treatment (risk ratio [RR] = 0.87; 95% CI, 0.77 to 0.99; absolute risk difference [ARD] = 10%; number needed to treat [NNT] = 10). No differences were observed based on the baclofen dose or duration of treatment. Baclofen treatment increased the number of abstinent days (mean difference [MD] = 9.07% higher; 95% CI, 3.30 to 14.85), with this effect observed at low and high doses. A greater duration of abstinence was associated with a longer treatment course (MD = 10.9; 95% CI, 3.17 to 18.62) and detoxification before baclofen initiation (MD = 11.76; 95% CI, 3.22 to 20.29). No significant differences were observed between baclofen and placebo in the number of heavy drinking days, drinks per drinking day, adverse events reported, and dropouts during treatment.
Compared with the placebo group, the baclofen group had an increased frequency of minor adverse events, including fatigue (RR = 1.31; 95% CI, 1.09 to 1.59; ARD = 7.7%; number needed to harm [NNH] = 12), dizziness (RR = 1.70; 95% CI, 1.35 to 2.13; ARD = 9.9%; NNH = 10), somnolence (RR = 1.36; 95% CI, 1.19 to 1.56; ARD = 10.1%; NNH = 9), dry mouth (RR = 1.79; 95% CI, 1.01 to 3.18; ARD = 2.78%; NNH = 35), and paresthesias (RR = 2.87; 95% CI, 1.17 to 7.06; ARD = 18.2%; NNH = 5). There was no difference in any other adverse events between baclofen and placebo.
Two trials compared baclofen with other alcohol use disorder treatments instead of placebo alone. No significant differences were observed between baclofen and acamprosate. It was unclear whether there were any differences between baclofen and naltrexone.
Caveats: There is moderate-certainty evidence for the use of baclofen compared with placebo; however, the evidence comparing baclofen with acamprosate and naltrexone is of very low certainty. Most trials included in the Cochrane review had small sample sizes, raising concerns about the generalizability of the results. The review included more men (70%) than women. The studies were conducted in locations including the United States, Europe, and Asia, which may affect results because social context can influence alcohol use disorder and associated treatment effects. There was significant heterogeneity in the trials overall, including the data and findings evaluating the potential adverse effects of baclofen compared with placebo. Study criteria frequently excluded participants with severe psychiatric disorders, which are often comorbid with alcohol use disorder, further limiting the generalizability of the findings.
Conclusion: The Cochrane review found moderate-certainty evidence to suggest that, compared with placebo, baclofen reduces the risk of relapse and increases the number of abstinent days in individuals with alcohol use disorder who have completed detoxification before treatment. Adverse effects associated with baclofen treatment include fatigue, dizziness, somnolence, dry mouth, and paresthesias. There is limited evidence comparing baclofen and other medications to treat alcohol use disorder. A single study suggests baclofen may be associated with increased rates of relapse compared with naltrexone, and no evidence was found to determine the differences between acamprosate and baclofen. Because of the moderate-certainty evidence in support of baclofen, the potential adverse events, and the study limitations, we have assigned a color recommendation of yellow (unclear benefits) for the use of baclofen to treat alcohol use disorder.
