
Am Fam Physician. 2023;108(4):408-410
Author disclosure: No relevant financial relationships.
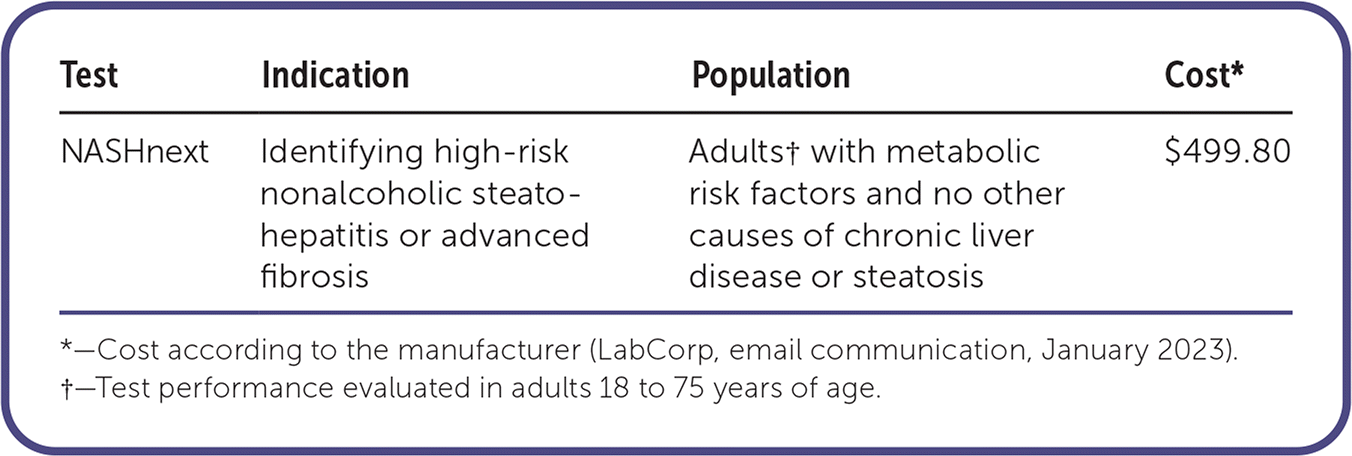
NASHnext is a noninvasive test that uses a proprietary blood-based diagnostic algorithm called NIS4 to identify nonalcoholic steato-hepatitis (NASH). It is recommended for patients with metabolic risk factors and no other causes of chronic liver disease or steatosis. NASHnext is one of many potential biomarkers for NASH. The NIS4 algorithm combines results from four independent NASH-associated biomarkers (miR-34a, a2M, YKL-40/CHI3L1, and A1C) into a score (0 to 1) that identifies those at risk of NASH and progression to advanced fibrosis (Table 1).1 It is projected that by 2030, NASH will be the leading cause of liver transplantation in the United States.2 The American Gastroenterological Association and several other national societies recommend noninvasive tests, such as Fibrosis-4, which also comprise biomarkers, to stratify a patient’s risk before performing imaging or liver biopsies.3
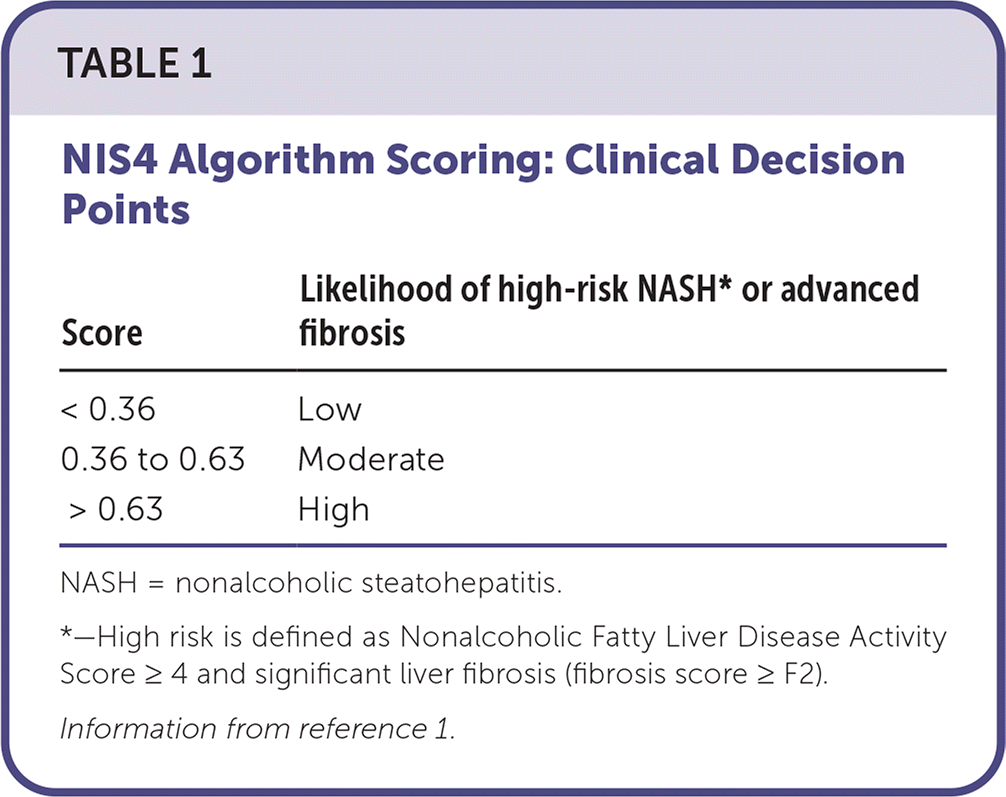
| Score | Likelihood of high-risk NASH* or advanced fibrosis |
|---|---|
| < 0.36 | Low |
| 0.36 to 0.63 | Moderate |
| > 0.63 | High |
Accuracy
No clinical trials have been completed to assess the impact of the NASHnext test on patient-centered outcomes. Using this test increases the risk of labeling effect rather than clinical diagnosis.
A 2020 industry-funded prospective validation study used blood samples, clinical data, and liver biopsy results from three independent cohorts with suspected nonalcoholic fatty liver disease (NAFLD) to develop and validate NIS4. Patients with biopsy-confirmed NASH who did not have cirrhosis were included (n = 239). Patients were excluded if daily alcohol consumption was more than two drinks per day (20 g) for women and three drinks per day (30 g) for men, steatohepatitis was due to a secondary cause, or any other chronic liver disease was identified. This discovery cohort was compared with two independent cohorts, comprising 475 patients and 227 patients, respectively, with suspected NAFLD and clinical risk factors for NASH (Table 2).4
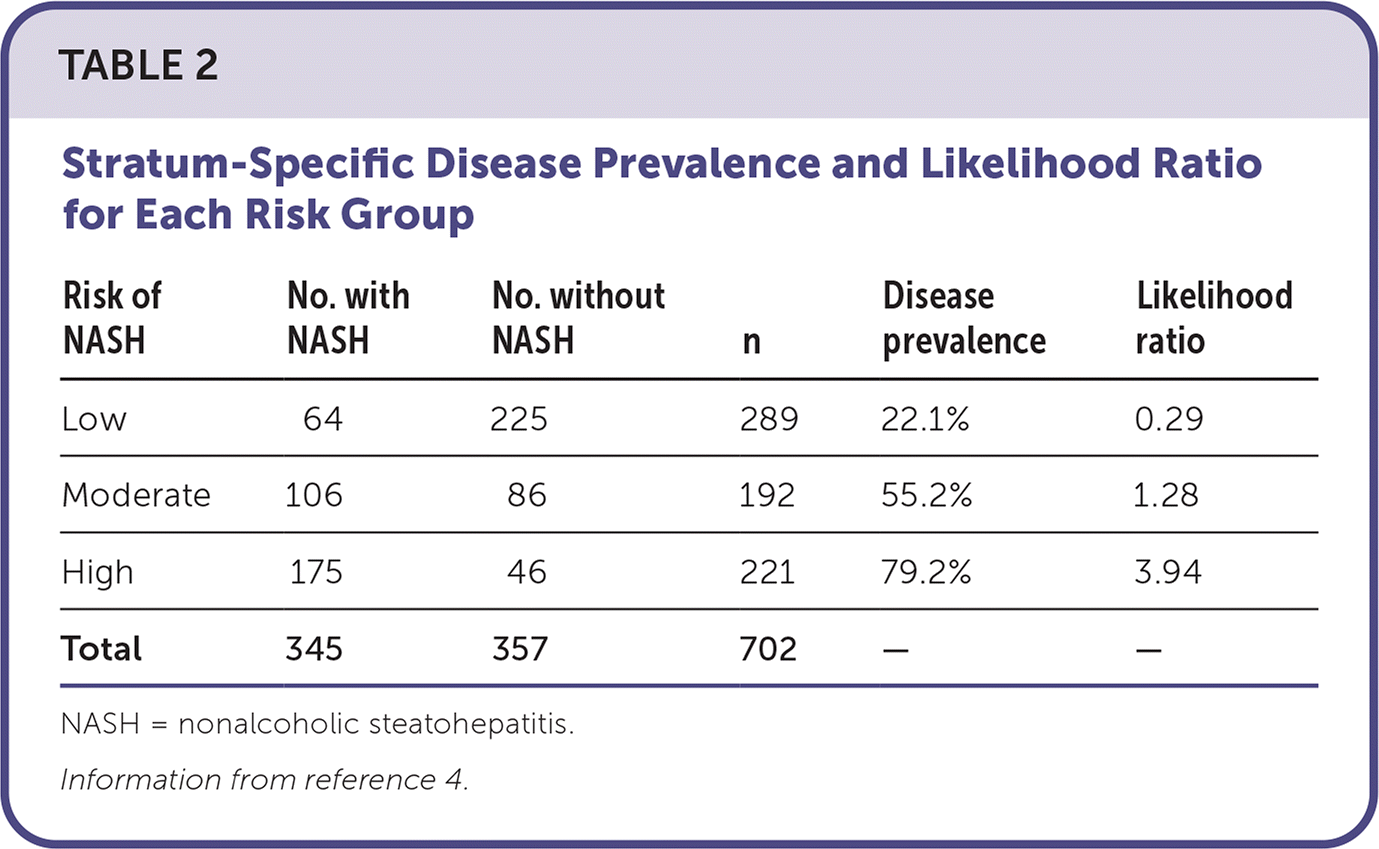
| Risk of NASH | No. with NASH | No. without NASH | n | Disease prevalence | Likelihood ratio |
|---|---|---|---|---|---|
| Low | 64 | 225 | 289 | 22.1% | 0.29 |
| Moderate | 106 | 86 | 192 | 55.2% | 1.28 |
| High | 175 | 46 | 221 | 79.2% | 3.94 |
| Total | 345 | 357 | 702 | — | — |
Benefit
NASHnext has the potential for noninvasively ruling in or out high-risk NASH, reducing the likelihood that liver biopsies will be performed in low-risk patients.4 Liver biopsy is the diagnostic standard for NAFLD. Although mortality from liver biopsy is low, the procedure can lead to serious complications such as major bleeding and patient stress.2 Early screening with noninvasive testing such as NASHnext could lead to discussions and shared decision-making regarding next steps in patients identified as having higher risk because NASH is often asymptomatic in early stages of the disease.5
Harms
NASHnext is not a diagnostic tool, and clinical trials are needed to confirm its validity. There is also the potential to falsely reassure patients with a low NASHnext score who do have NASH, and these patients may not receive appropriate liver biopsy or a diagnosis based on the score. In a low-risk study group, 22% of patients had NASH.4 The NASHnext test has not yet been approved by the U.S. Food and Drug Administration.5 Tests have not been validated in children, and it is unclear whether patients with an abnormal NASH-next test result are at higher risk of disease progression.
Cost
Use of NASHnext (owned by LabCorp, test 504960) can be billed to insurance at $499.80, but it is not routinely covered by health insurance. The out-of-pocket cost to patients is $411.08.6 Liver biopsy (Current Procedural Terminology [CPT] code 4700) in ambulatory surgical centers costs approximately $600 per Medicare, and the patient cost is about $140. Testing at hospital outpatient departments costs approximately $1,300, with an out-of-pocket cost to patients of around $300.7
Bottom Line
NASHnext is a proposed noninvasive test aiming to rule in or out metabolic risk factors for NASH or advanced fibrosis, but further clinical trials are needed for validation. Future trials comparing Fibrosis-4 with NASHnext are also needed before NASHnext can be recommended as a noninvasive method to assess for hepatic fibrosis. Currently, the test should not replace referral for a liver biopsy, which remains the preferred method for NASH diagnosis and staging. If validated, NASHnext could provide a diagnostic approach in the primary care setting.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army, the U.S. Department of Defense, or the U.S. government.
