
Am Fam Physician. 2023;108(5):online
Author disclosure: No relevant financial relationships.

Details for This Review
Study Population: 5,831 patients with depression and a history of stroke
Efficacy End Points: Remission of depression at the end of treatment; inadequate response to treatment (less than 50% reduction in scale scores at the end of treatment)
Harm End Points: Adverse events from therapy such as death, neurologic events, and gastrointestinal effects
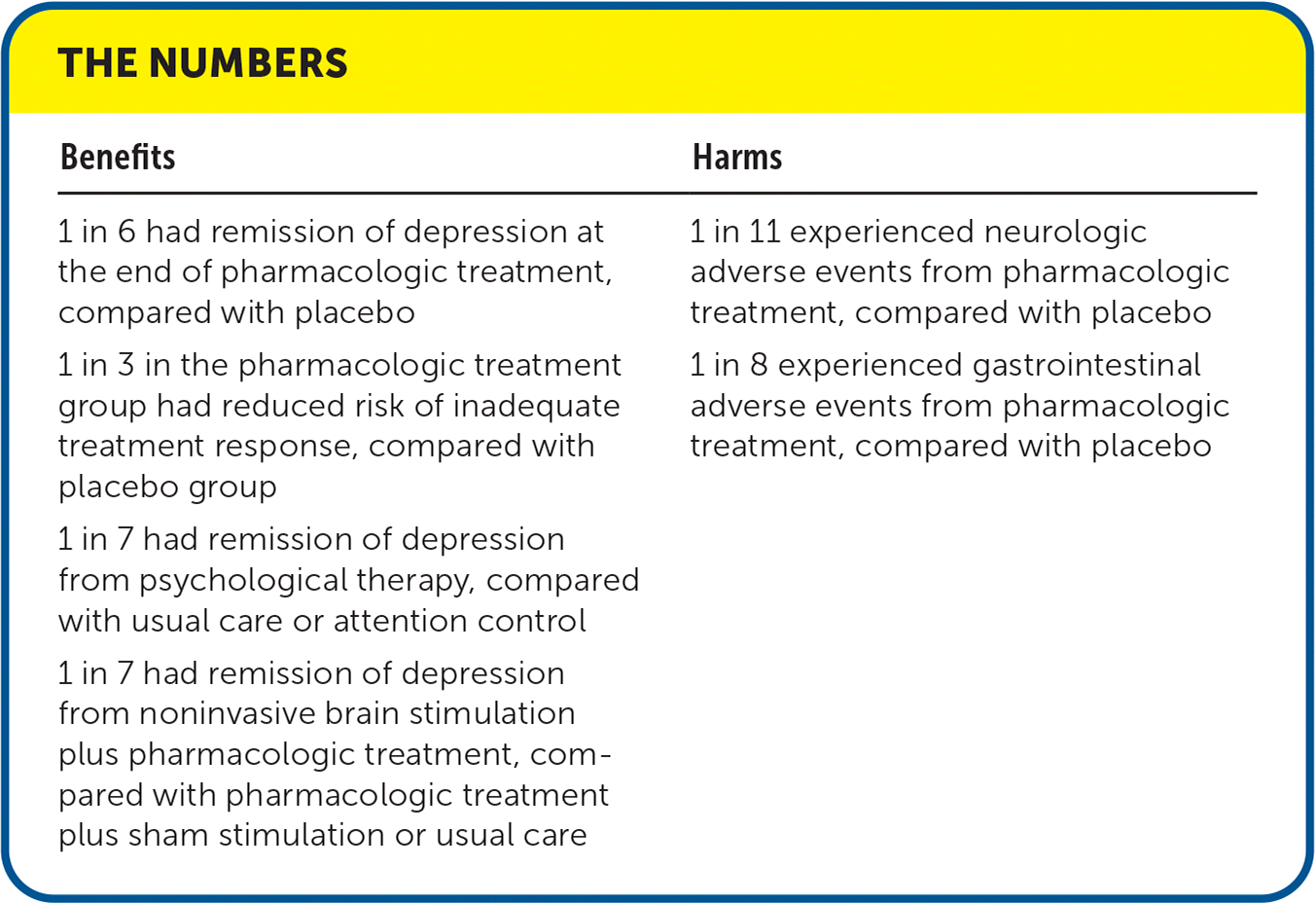
| Benefits | Harms |
|---|---|
| 1 in 6 had remission of depression at the end of pharmacologic treatment, compared with placebo 1 in 3 in the pharmacologic treatment group had reduced risk of inadequate treatment response, compared with placebo group 1 in 7 had remission of depression from psychological therapy, compared with usual care or attention control 1 in 7 had remission of depression from noninvasive brain stimulation plus pharmacologic treatment, compared with pharmacologic treatment plus sham stimulation or usual care | 1 in 11 experienced neurologic adverse events from pharmacologic treatment, compared with placebo 1 in 8 experienced gastrointestinal adverse events from pharmacologic treatment, compared with placebo |
Narrative: Approximately one-third of stroke survivors experience depression within five years of the stroke.1,2 Poststroke depression is associated with increased mortality and can negatively impact activities of daily living and cognitive function.3–5 Given the U.S. Preventive Services Task Force recommendation for routine depression screening in adults, family physicians are well positioned to identify and treat poststroke depression in the ambulatory setting.6 The main treatment options for poststroke depression include pharmacologic therapy, noninvasive brain stimulation, and psychological interventions.
A 2023 Cochrane review evaluated multiple treatment modalities for poststroke depression.7 The review included 65 randomized controlled trials (RCTs), with 5,831 participants who had a history of stroke and a diagnosis of depression on recruitment. The studied interventions were pharmacologic therapy (18 trials with 1,829 participants; 12 trials studied selective serotonin reuptake inhibitors, and two trials studied tricyclic antidepressants), noninvasive brain stimulation (eight trials with 516 participants), psychological interventions (22 trials with 1,764 participants), or a combination (three trials with 278 participants). The mean age of participants ranged from 52 to 78 years. The period between stroke diagnosis and depression intervention varied from within one week to more than one year. In this review, stroke included cerebral infarction, intracerebral hemorrhage, and uncertain pathologic subtypes. The primary outcomes were remission of depression at the end of treatment (using the Diagnostic and Statistical Manual of Mental Disorders, 5th ed, criteria for depression or similar standard diagnostic criteria), inadequate response to treatment (less than a 50% reduction in scale scores at the end of treatment), and adverse events.
Very low-certainty evidence showed that, compared with placebo, pharmacologic interventions are more likely to achieve remission of depression after a treatment course of varying duration (risk ratio [RR] = 0.70; 95% CI, 0.55 to 0.88; absolute risk difference [ARD] = 17.9%; number needed to treat [NNT] = 6; eight RCTs with 1,025 participants) and have a lower likelihood of inadequate depression treatment response (RR = 0.48; 95% CI, 0.32 to 0.70; ARD = 37.7%; NNT = 3; six RCTs with 511 participants). Very low-certainty evidence suggested that, compared with usual care or attention control, psychological therapy is more likely to achieve remission of depression (RR = 0.77; 95% CI, 0.62 to 0.95; ARD = 16.2%; NNT = 7; six trials with 521 participants). The combination of pharmacologic therapy plus noninvasive brain stimulation is more likely to achieve remission of depression vs. pharmacologic therapy plus sham stimulation or usual care (RR = 0.77; 95% CI, 0.64 to 0.91; ARD = 14.7%; NNT = 7; three RCTs with 392 participants; low-certainty evidence).
Very low-certainty evidence demonstrated that, compared with placebo, the pharmacologic treatment group had more neurologic adverse events (RR = 1.55; 95% CI, 1.12 to 2.15; ARD = 8.5%; number needed to harm [NNH] = 11) and gastrointestinal adverse events (RR = 1.62; 95% CI, 1.19 to 2.19; ARD = 11.2%; NNH = 8), although pharmacologic treatment did not increase mortality rates. The analysis found no difference in adverse events for patients treated with noninvasive brain stimulation, psychological therapy, or a combination of these, compared with placebo or usual care.
Caveats: The Cochrane review had several limitations. Many trials excluded participants with communication problems, cognitive impairment, concomitant traumatic brain injuries, or psychiatric conditions, which can be common among stroke survivors, limiting generalizability. Depression treatment guidelines from the U.S. Department of Veterans Affairs and U.S. Department of Defense recommend that patients take antidepressants for at least six months before an adequate treatment assessment can be performed.8 Because most trials included a shorter pharmacologic treatment period, this review may have inadequately assessed the effectiveness of antidepressants. Many trials showed substantial heterogeneity (I2 = 50% to 89%) and had high risk of bias in multiple domains (e.g., allocation concealment [selection bias], blinding [performance bias], and incomplete outcome data [attrition bias]). Diagnostic criteria for depression were also inconsistent among trials.
Conclusion: Although the results are promising for pharmacologic and psychological therapies and for the combination of noninvasive brain stimulation plus pharmacologic therapies, we assigned a color recommendation of yellow (unclear benefits) for the treatment of poststroke depression based on a high potential for bias, significant heterogeneity, and limited generalizability. Further research with longer duration of antidepressant therapy is needed to properly evaluate the balance of benefits and risks. Additional studies are also needed to investigate the effect of treatment in stroke survivors with communication barriers, traumatic brain injury, or concomitant psychiatric disorders.
