
Am Fam Physician. 2023;108(6):online
Author disclosure: No relevant financial relationships.
Details for This Review
Study Population: 1,689 adult patients with severe community-acquired pneumonia (CAP) from seven randomized controlled trials (RCTs) comparing adjunctive corticosteroids with placebo or standard care
Efficacy End Points: 30-day, all-cause mortality; requirement for mechanical ventilation; length of intensive care unit (ICU) stay; length of hospital stay
Harm End Points: Adverse events, including gastrointestinal bleeding, health care–associated infection, acute kidney injury, and hospital readmission
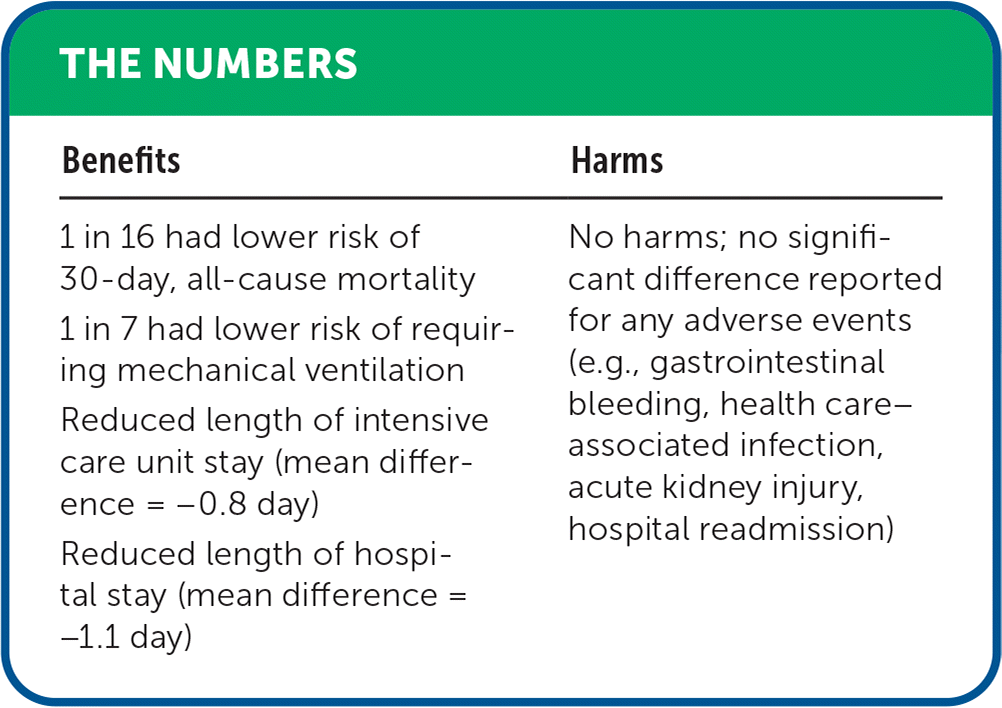
| Benefits | Harms |
|---|---|
| 1 in 16 had lower risk of 30-day, all-cause mortality | No harms; no significant difference reported for any adverse events (e.g., gastrointestinal bleeding, health care–associated infection, acute kidney injury, hospital readmission) |
| 1 in 7 had lower risk of requiring mechanical ventilation | |
| Reduced length of intensive care unit stay (mean difference = −0.8 day) | |
| Reduced length of hospital stay (mean difference = −1.1 day) |
Narrative: Severe CAP is a leading cause of sepsis, hospitalization, and mortality, particularly in older patients, those with significant comorbidities, and those who are immunocompromised1–4 (Table 13). Primary treatment for CAP involves administering antimicrobials, and several RCTs have suggested that adjunctive corticosteroids may improve patient outcomes in those with severe CAP.5–10 However, prior systematic reviews and meta-analyses have not always included patients with severe CAP, and results have varied.11–13 One 2023 RCT investigating patients with severe CAP demonstrated reduced 28-day mortality with corticosteroids compared with placebo,14 which was not demonstrated in prior meta-analyses. Current guidelines differ from previous recommendations regarding the use of adjunctive corticosteroids in patients with severe CAP.3,4,15
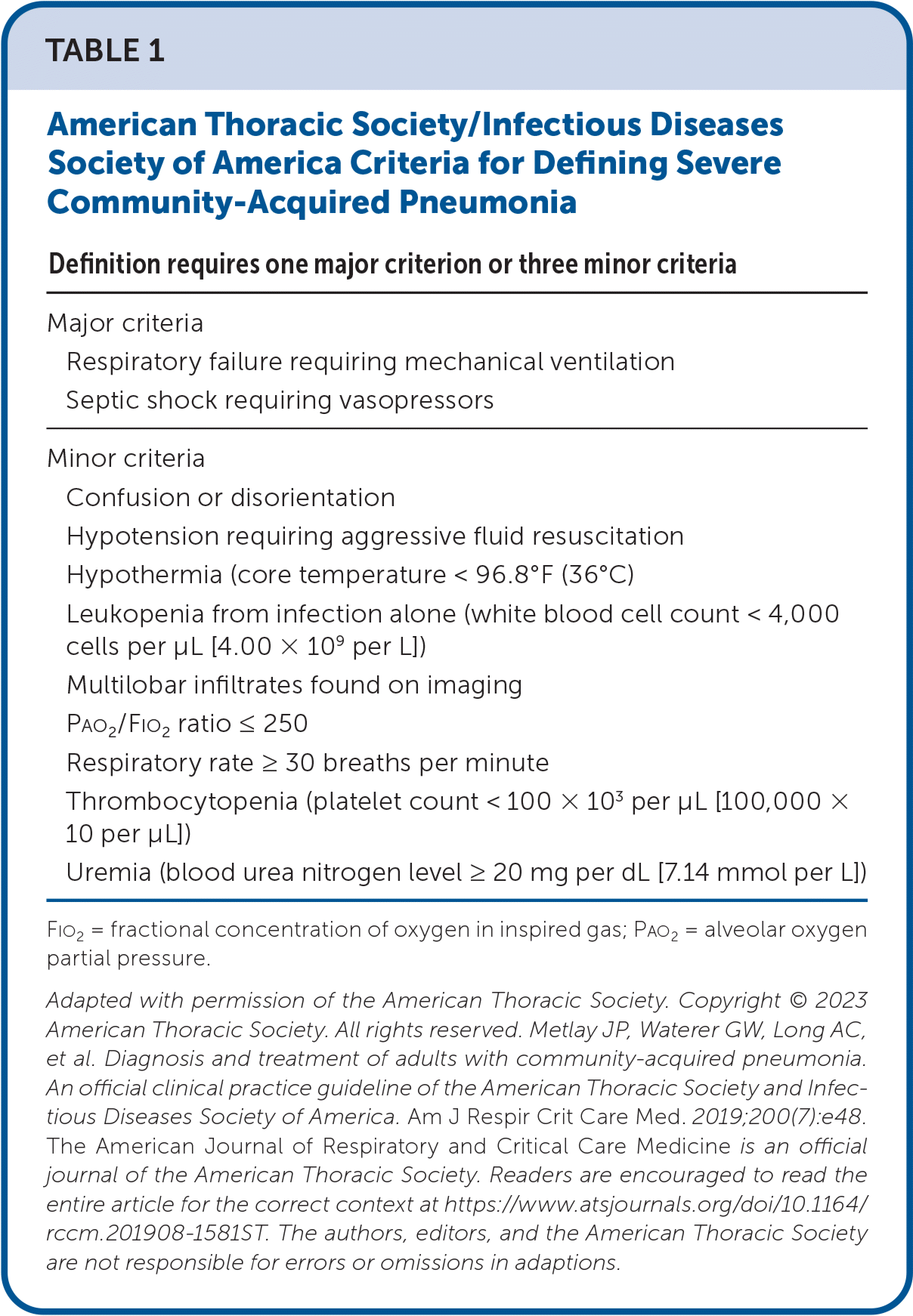
| Definition requires one major criterion or three minor criteria |
|---|
| Major criteria |
| Respiratory failure requiring mechanical ventilation |
| Septic shock requiring vasopressors |
| Minor criteria |
| Confusion or disorientation |
| Hypotension requiring aggressive fluid resuscitation |
| Hypothermia (core temperature < 96.8°F (36°C) |
| Leukopenia from infection alone (white blood cell count < 4,000 cells per μL [4.00 × 109 per L]) |
| Multilobar infiltrates found on imaging |
| Pao2/Fio2 ratio ≤ 250 |
| Respiratory rate ≥ 30 breaths per minute |
| Thrombocytopenia (platelet count < 100 × 103 per μL [100,000 × 10 per μL]) |
| Uremia (blood urea nitrogen level ≥ 20 mg per dL [7.14 mmol per L]) |
A 2023 meta-analysis incorporates recent trials and evaluates the use of adjunctive corticosteroids compared with placebo or usual care in adults with severe CAP.16 This meta-analysis included seven RCTs and 1,689 patients.16 The seven RCTs were double-blinded; five of those were multicenter. Two of the trials were completed in the United States, and the others were completed in Egypt, France, Italy, Saudi Arabia, and Spain. The studies compared systemic corticosteroids of any type, dosage, and duration with placebo or standard care. Severe CAP was defined as the patient requiring admission to the intensive care unit for pneumonia that is classified as severe based on the American Thoracic Society/Infectious Diseases Society of America guidelines (Table 13) or as risk class V based on the Pneumonia Severity Index.3,4,17 The primary outcome included 30-day, all-cause mortality. The secondary outcomes included the need for mechanical ventilation, length of ICU stay, length of hospital stay, and adverse events (e.g., gastrointestinal bleeding, health care–associated infection, acute kidney injury, hospital readmission).
Compared with the control treatment, adjunctive corticosteroids reduced 30-day, all-cause mortality (risk ratio = 0.61; 95% CI, 0.44 to 0.85; absolute risk difference = 6.3%; number needed to treat = 16; seven RCTs; N = 1,689) and the need for mechanical ventilation (risk ratio = 0.57; 95% CI, 0.45 to 0.73; absolute risk difference = 14.9%; number needed to treat = 7; five RCTs; N = 718). Adjunctive corticosteroids were also associated with a shorter ICU stay (mean difference = −0.8 days; 95% CI, −1.4 to −0.1; five RCTs; N = 1,261) and hospital stay (mean difference = −1.1 days; 95% CI, −2.0 to −0.1; three RCTs; N = 750). There was no reported difference in gastrointestinal bleeding, health care–associated infection, acute kidney injury, or hospital readmission.16
The results of this meta-analysis are similar to results of a 2017 Cochrane review (17 RCTs with 2,264 total patients and 13 RCTs with 1,954 total patients) examining corticosteroids for the treatment of hospitalized adults with pneumonia.12 The 2017 review also demonstrated a reduction in morbidity and mortality in inpatients with severe CAP. However, it demonstrated a higher incidence of adverse effects, specifically hyperglycemia, in patients who received corticosteroids.12
Caveats: The 2023 meta-analysis has several limitations.16 First, two recent studies account for most included patients.9,14 The largest of these two studies was the only RCT to demonstrate statistically significant reduction in all-cause mortality.14 Although the definition of severe CAP varied in the included studies, subgroup analysis of outcomes based on definitions demonstrated similar findings. Additionally, the corticosteroid type, dosage, regimen, and duration of treatment varied significantly across studies. Although the optimal dosage and duration of corticosteroids are unclear, the study that demonstrated reduction in all-cause mortality used hydrocortisone, 200 mg per day, for four or eight days.14 Three RCTs had risk of bias based on the randomization process, and one RCT had risk of bias based on outcome measurement. Finally, the evaluation of adverse outcomes was underpowered.
Conclusion: Despite several limitations, the 2023 meta-analysis found that using adjunctive corticosteroids in adults with severe CAP reduced 30-day, all-cause mortality; need for mechanical ventilation; length of ICU stay; and length of hospital stay with no increase in adverse events.16 Because the benefits are greater than the harms, we have assigned a color recommendation of green to this treatment. However, it must be noted that the specific dosage and duration of corticosteroid therapy remain unclear. Further studies are needed to compare individual corticosteroid types, durations, and dosages.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Department of Defense, the U.S. government, the Defense Health Agency, U.S. Army, U.S. Air Force, or Brooke Army Medical Center.
